Christian Daul
Evaluation of Few-Shot Learning Methods for Kidney Stone Type Recognition in Ureteroscopy
May 23, 2025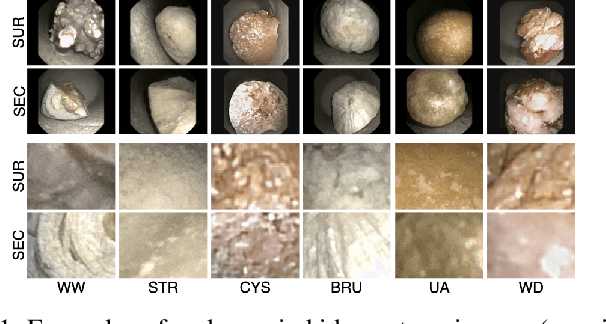
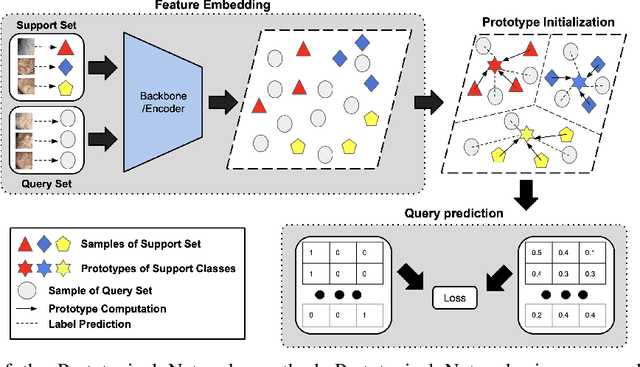
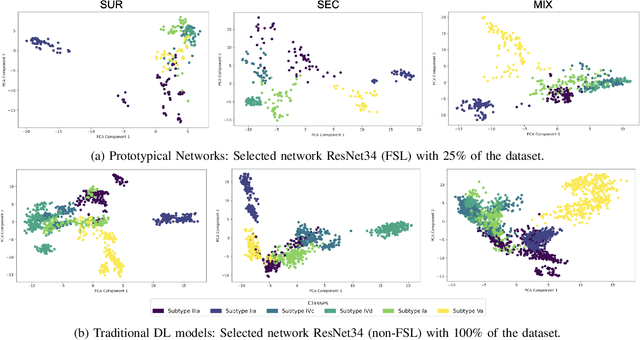
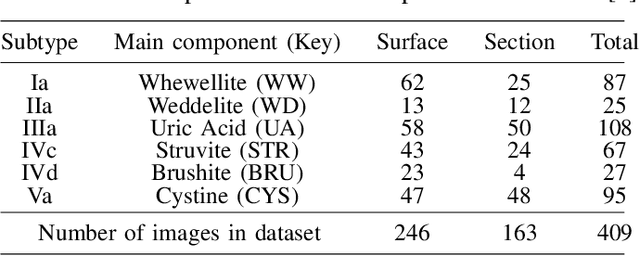
Abstract:Determining the type of kidney stones is crucial for prescribing appropriate treatments to prevent recurrence. Currently, various approaches exist to identify the type of kidney stones. However, obtaining results through the reference ex vivo identification procedure can take several weeks, while in vivo visual recognition requires highly trained specialists. For this reason, deep learning models have been developed to provide urologists with an automated classification of kidney stones during ureteroscopies. Nevertheless, a common issue with these models is the lack of training data. This contribution presents a deep learning method based on few-shot learning, aimed at producing sufficiently discriminative features for identifying kidney stone types in endoscopic images, even with a very limited number of samples. This approach was specifically designed for scenarios where endoscopic images are scarce or where uncommon classes are present, enabling classification even with a limited training dataset. The results demonstrate that Prototypical Networks, using up to 25% of the training data, can achieve performance equal to or better than traditional deep learning models trained with the complete dataset.
Assessing the generalization performance of SAM for ureteroscopy scene understanding
May 22, 2025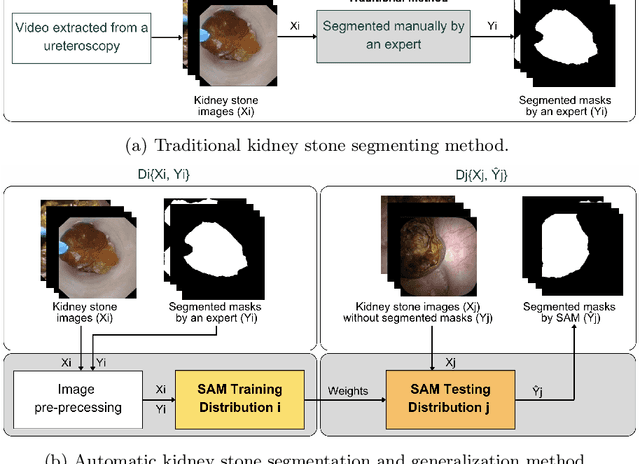
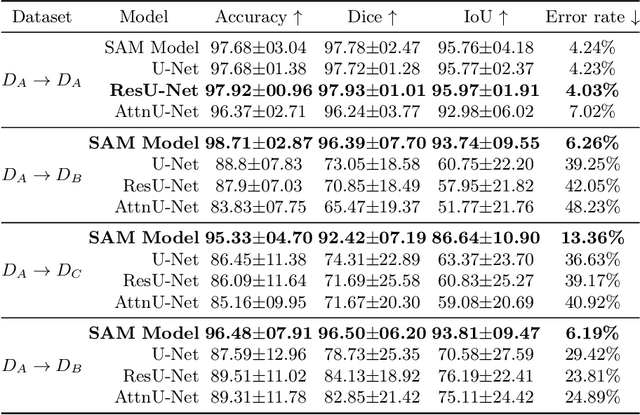
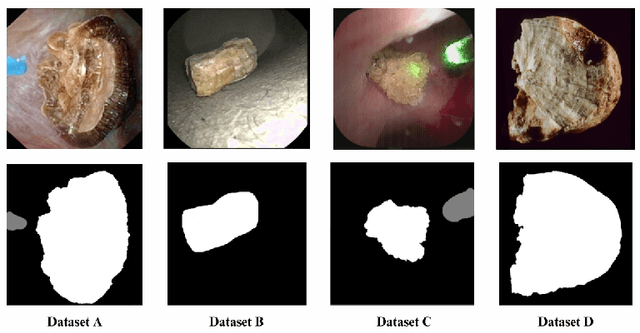
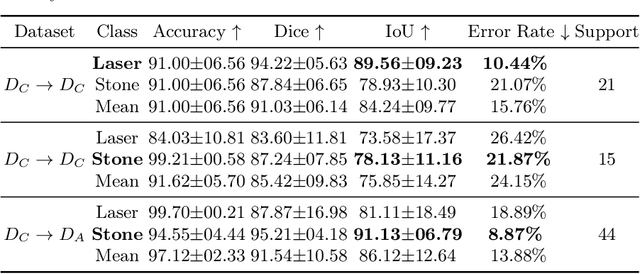
Abstract:The segmentation of kidney stones is regarded as a critical preliminary step to enable the identification of urinary stone types through machine- or deep-learning-based approaches. In urology, manual segmentation is considered tedious and impractical due to the typically large scale of image databases and the continuous generation of new data. In this study, the potential of the Segment Anything Model (SAM) -- a state-of-the-art deep learning framework -- is investigated for the automation of kidney stone segmentation. The performance of SAM is evaluated in comparison to traditional models, including U-Net, Residual U-Net, and Attention U-Net, which, despite their efficiency, frequently exhibit limitations in generalizing to unseen datasets. The findings highlight SAM's superior adaptability and efficiency. While SAM achieves comparable performance to U-Net on in-distribution data (Accuracy: 97.68 + 3.04; Dice: 97.78 + 2.47; IoU: 95.76 + 4.18), it demonstrates significantly enhanced generalization capabilities on out-of-distribution data, surpassing all U-Net variants by margins of up to 23 percent.
Leveraging Pre-trained Models for Robust Federated Learning for Kidney Stone Type Recognition
Sep 30, 2024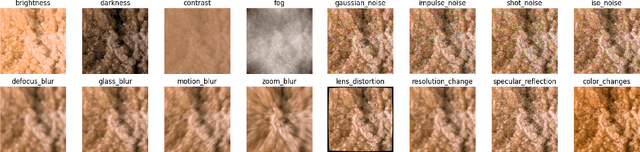
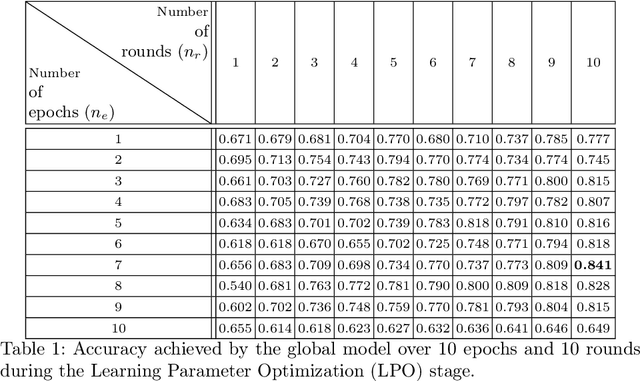
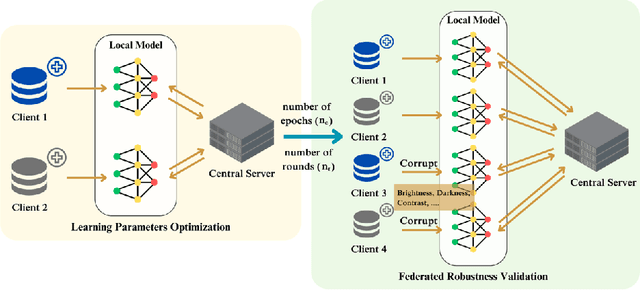
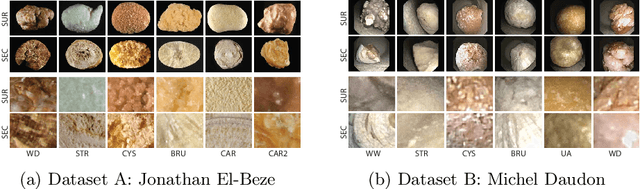
Abstract:Deep learning developments have improved medical imaging diagnoses dramatically, increasing accuracy in several domains. Nonetheless, obstacles continue to exist because of the requirement for huge datasets and legal limitations on data exchange. A solution is provided by Federated Learning (FL), which permits decentralized model training while maintaining data privacy. However, FL models are susceptible to data corruption, which may result in performance degradation. Using pre-trained models, this research suggests a strong FL framework to improve kidney stone diagnosis. Two different kidney stone datasets, each with six different categories of images, are used in our experimental setting. Our method involves two stages: Learning Parameter Optimization (LPO) and Federated Robustness Validation (FRV). We achieved a peak accuracy of 84.1% with seven epochs and 10 rounds during LPO stage, and 77.2% during FRV stage, showing enhanced diagnostic accuracy and robustness against image corruption. This highlights the potential of merging pre-trained models with FL to address privacy and performance concerns in medical diagnostics, and guarantees improved patient care and enhanced trust in FL-based medical systems.
EndoDepth: A Benchmark for Assessing Robustness in Endoscopic Depth Prediction
Sep 30, 2024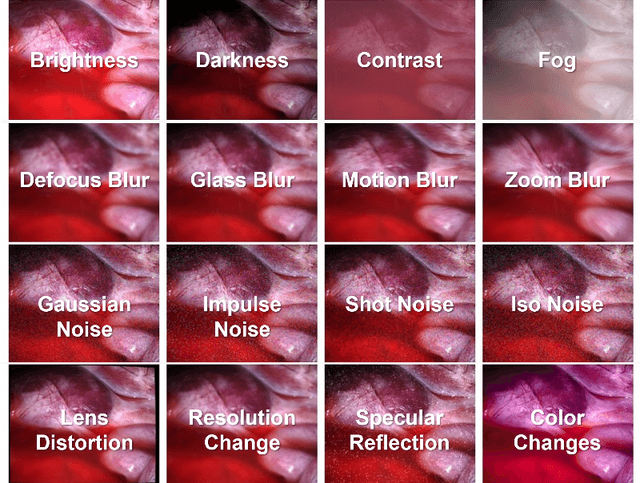
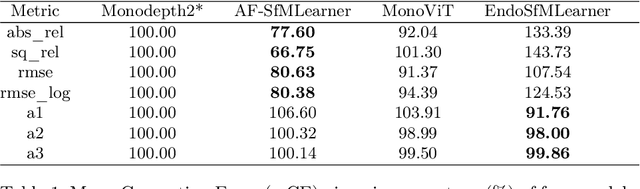
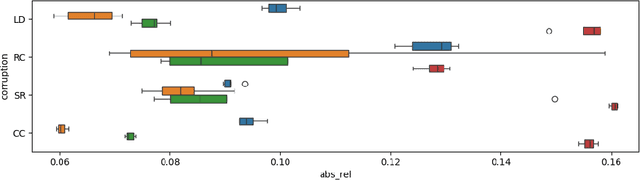

Abstract:Accurate depth estimation in endoscopy is vital for successfully implementing computer vision pipelines for various medical procedures and CAD tools. In this paper, we present the EndoDepth benchmark, an evaluation framework designed to assess the robustness of monocular depth prediction models in endoscopic scenarios. Unlike traditional datasets, the EndoDepth benchmark incorporates common challenges encountered during endoscopic procedures. We present an evaluation approach that is consistent and specifically designed to evaluate the robustness performance of the model in endoscopic scenarios. Among these is a novel composite metric called the mean Depth Estimation Robustness Score (mDERS), which offers an in-depth evaluation of a model's accuracy against errors brought on by endoscopic image corruptions. Moreover, we present SCARED-C, a new dataset designed specifically to assess endoscopy robustness. Through extensive experimentation, we evaluate state-of-the-art depth prediction architectures on the EndoDepth benchmark, revealing their strengths and weaknesses in handling endoscopic challenging imaging artifacts. Our results demonstrate the importance of specialized techniques for accurate depth estimation in endoscopy and provide valuable insights for future research directions.
A metric learning approach for endoscopic kidney stone identification
Jul 13, 2023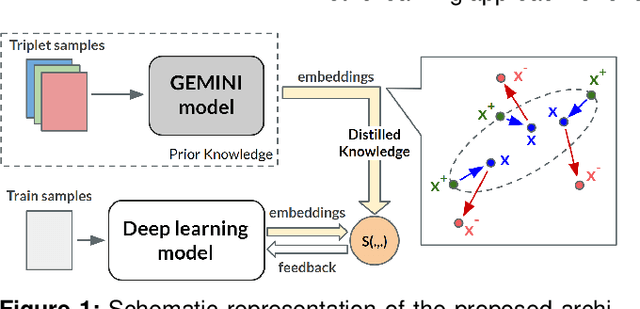
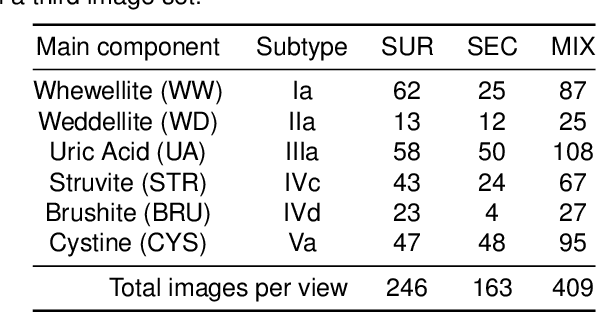
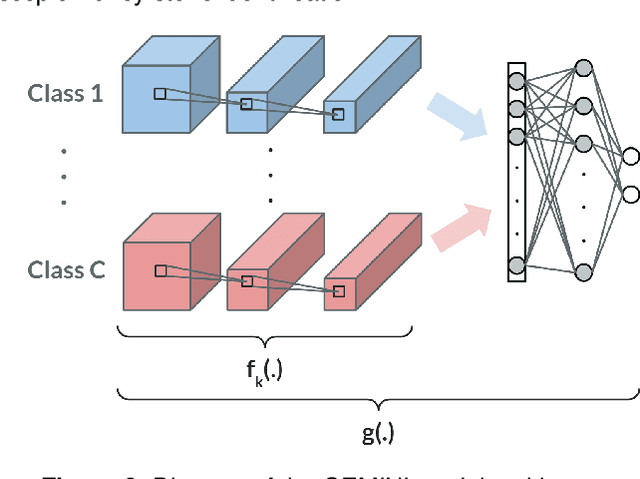
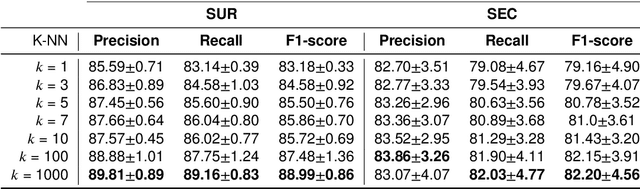
Abstract:Several Deep Learning (DL) methods have recently been proposed for an automated identification of kidney stones during an ureteroscopy to enable rapid therapeutic decisions. Even if these DL approaches led to promising results, they are mainly appropriate for kidney stone types for which numerous labelled data are available. However, only few labelled images are available for some rare kidney stone types. This contribution exploits Deep Metric Learning (DML) methods i) to handle such classes with few samples, ii) to generalize well to out of distribution samples, and iii) to cope better with new classes which are added to the database. The proposed Guided Deep Metric Learning approach is based on a novel architecture which was designed to learn data representations in an improved way. The solution was inspired by Few-Shot Learning (FSL) and makes use of a teacher-student approach. The teacher model (GEMINI) generates a reduced hypothesis space based on prior knowledge from the labeled data, and is used it as a guide to a student model (i.e., ResNet50) through a Knowledge Distillation scheme. Extensive tests were first performed on two datasets separately used for the recognition, namely a set of images acquired for the surfaces of the kidney stone fragments, and a set of images of the fragment sections. The proposed DML-approach improved the identification accuracy by 10% and 12% in comparison to DL-methods and other DML-approaches, respectively. Moreover, model embeddings from the two dataset types were merged in an organized way through a multi-view scheme to simultaneously exploit the information of surface and section fragments. Test with the resulting mixed model improves the identification accuracy by at least 3% and up to 30% with respect to DL-models and shallow machine learning methods, respectively.
Deep learning-based image exposure enhancement as a pre-processing for an accurate 3D colon surface reconstruction
Apr 14, 2023



Abstract:This contribution shows how an appropriate image pre-processing can improve a deep-learning based 3D reconstruction of colon parts. The assumption is that, rather than global image illumination corrections, local under- and over-exposures should be corrected in colonoscopy. An overview of the pipeline including the image exposure correction and a RNN-SLAM is first given. Then, this paper quantifies the reconstruction accuracy of the endoscope trajectory in the colon with and without appropriate illumination correction
Deep Prototypical-Parts Ease Morphological Kidney Stone Identification and are Competitively Robust to Photometric Perturbations
Apr 08, 2023Abstract:Identifying the type of kidney stones can allow urologists to determine their cause of formation, improving the prescription of appropriate treatments to diminish future relapses. Currently, the associated ex-vivo diagnosis (known as Morpho-constitutional Analysis, MCA) is time-consuming, expensive and requires a great deal of experience, as it requires a visual analysis component that is highly operator dependant. Recently, machine learning methods have been developed for in-vivo endoscopic stone recognition. Deep Learning (DL) based methods outperform non-DL methods in terms of accuracy but lack explainability. Despite this trade-off, when it comes to making high-stakes decisions, it's important to prioritize understandable Computer-Aided Diagnosis (CADx) that suggests a course of action based on reasonable evidence, rather than a model prescribing a course of action. In this proposal, we learn Prototypical Parts (PPs) per kidney stone subtype, which are used by the DL model to generate an output classification. Using PPs in the classification task enables case-based reasoning explanations for such output, thus making the model interpretable. In addition, we modify global visual characteristics to describe their relevance to the PPs and the sensitivity of our model's performance. With this, we provide explanations with additional information at the sample, class and model levels in contrast to previous works. Although our implementation's average accuracy is lower than state-of-the-art (SOTA) non-interpretable DL models by 1.5 %, our models perform 2.8% better on perturbed images with a lower standard deviation, without adversarial training. Thus, Learning PPs has the potential to create more robust DL models.
Improving automatic endoscopic stone recognition using a multi-view fusion approach enhanced with two-step transfer learning
Apr 06, 2023Abstract:This contribution presents a deep-learning method for extracting and fusing image information acquired from different viewpoints, with the aim to produce more discriminant object features for the identification of the type of kidney stones seen in endoscopic images. The model was further improved with a two-step transfer learning approach and by attention blocks to refine the learned feature maps. Deep feature fusion strategies improved the results of single view extraction backbone models by more than 6% in terms of accuracy of the kidney stones classification.
Improved Kidney Stone Recognition Through Attention and Multi-View Feature Fusion Strategies
Nov 05, 2022Abstract:This contribution presents a deep learning method for the extraction and fusion of information relating to kidney stone fragments acquired from different viewpoints of the endoscope. Surface and section fragment images are jointly used during the training of the classifier to improve the discrimination power of the features by adding attention layers at the end of each convolutional block. This approach is specifically designed to mimic the morpho-constitutional analysis performed in ex-vivo by biologists to visually identify kidney stones by inspecting both views. The addition of attention mechanisms to the backbone improved the results of single view extraction backbones by 4% on average. Moreover, in comparison to the state-of-the-art, the fusion of the deep features improved the overall results up to 11% in terms of kidney stone classification accuracy.
Multi-Scale Structural-aware Exposure Correction for Endoscopic Imaging
Oct 26, 2022



Abstract:Endoscopy is the most widely used imaging technique for the diagnosis of cancerous lesions in hollow organs. However, endoscopic images are often affected by illumination artefacts: image parts may be over- or underexposed according to the light source pose and the tissue orientation. These artifacts have a strong negative impact on the performance of computer vision or AI-based diagnosis tools. Although endoscopic image enhancement methods are greatly required, little effort has been devoted to over- and under-exposition enhancement in real-time. This contribution presents an extension to the objective function of LMSPEC, a method originally introduced to enhance images from natural scenes. It is used here for the exposure correction in endoscopic imaging and the preservation of structural information. To the best of our knowledge, this contribution is the first one that addresses the enhancement of endoscopic images using deep learning (DL) methods. Tested on the Endo4IE dataset, the proposed implementation has yielded a significant improvement over LMSPEC reaching a SSIM increase of 4.40% and 4.21% for over- and underexposed images, respectively.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge