Caspar Gruijthuijsen
Bayesian grey-box identification of nonlinear convection effects in heat transfer dynamics
Jul 01, 2024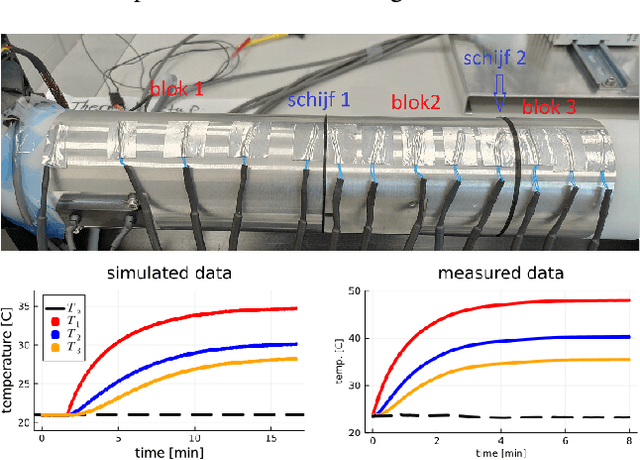
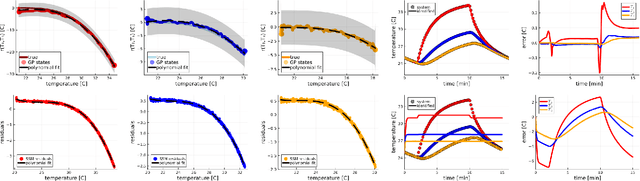
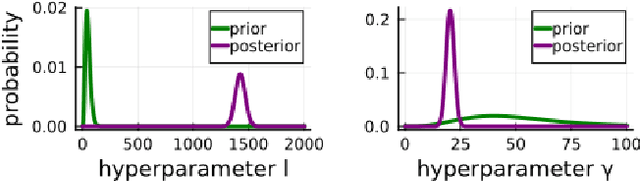
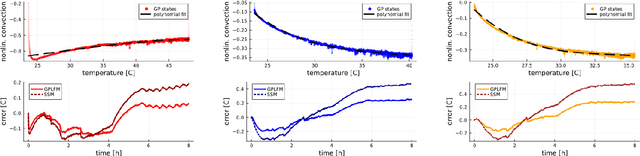
Abstract:We propose a computational procedure for identifying convection in heat transfer dynamics. The procedure is based on a Gaussian process latent force model, consisting of a white-box component (i.e., known physics) for the conduction and linear convection effects and a Gaussian process that acts as a black-box component for the nonlinear convection effects. States are inferred through Bayesian smoothing and we obtain approximate posterior distributions for the kernel covariance function's hyperparameters using Laplace's method. The nonlinear convection function is recovered from the Gaussian process states using a Bayesian regression model. We validate the procedure by simulation error using the identified nonlinear convection function, on both data from a simulated system and measurements from a physical assembly.
Autonomous Robotic Endoscope Control based on Semantically Rich Instructions
Jul 05, 2021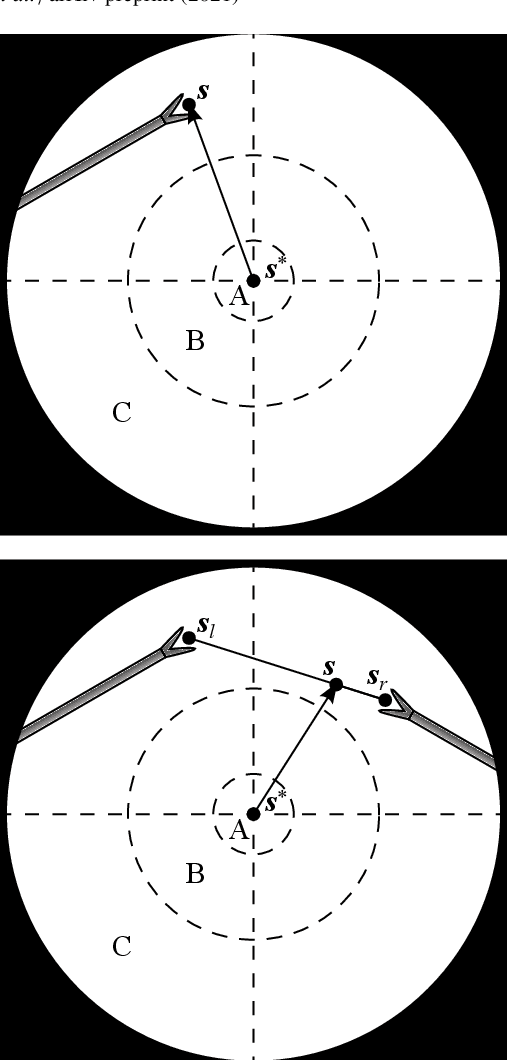
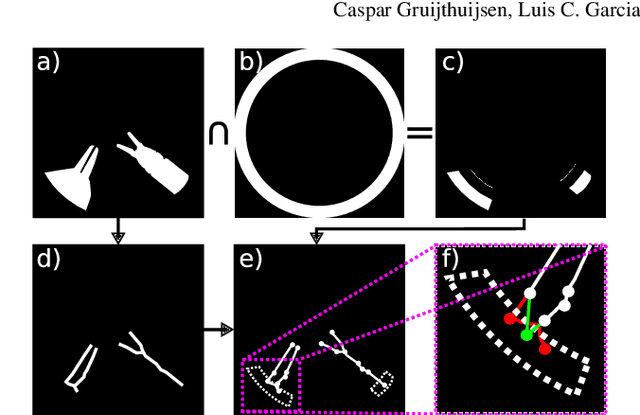
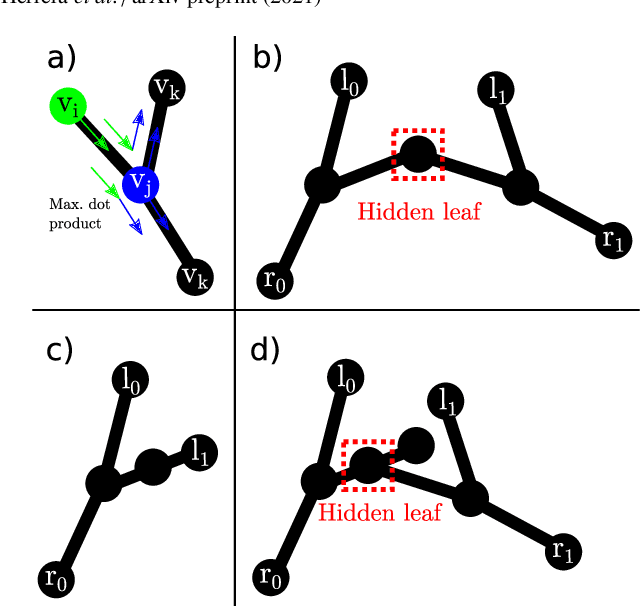
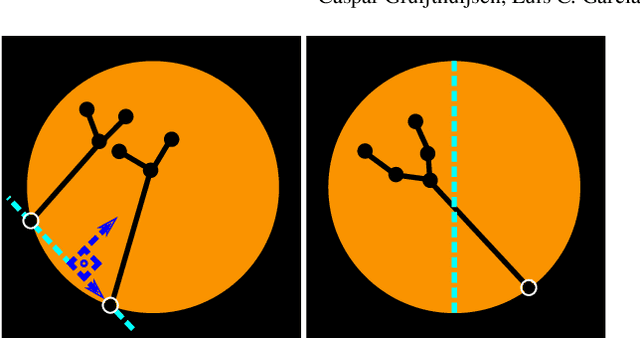
Abstract:In keyhole interventions, surgeons rely on a colleague to act as a camera assistant when their hands are occupied with surgical instruments. This often leads to reduced image stability, increased task completion times and sometimes errors. Robotic endoscope holders (REHs), controlled by a set of basic instructions, have been proposed as an alternative, but their unnatural handling increases the cognitive load of the surgeon, hindering their widespread clinical acceptance. We propose that REHs collaborate with the operating surgeon via semantically rich instructions that closely resemble those issued to a human camera assistant, such as "focus on my right-hand instrument". As a proof-of-concept, we present a novel system that paves the way towards a synergistic interaction between surgeons and REHs. The proposed platform allows the surgeon to perform a bi-manual coordination and navigation task, while a robotic arm autonomously performs various endoscope positioning tasks. Within our system, we propose a novel tooltip localization method based on surgical tool segmentation, and a novel visual servoing approach that ensures smooth and correct motion of the endoscope camera. We validate our vision pipeline and run a user study of this system. Through successful application in a medically proven bi-manual coordination and navigation task, the framework has shown to be a promising starting point towards broader clinical adoption of REHs.
Real-Time Segmentation of Non-Rigid Surgical Tools based on Deep Learning and Tracking
Sep 07, 2020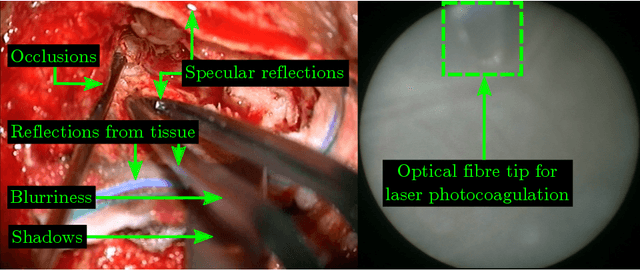
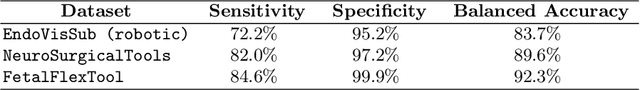
Abstract:Real-time tool segmentation is an essential component in computer-assisted surgical systems. We propose a novel real-time automatic method based on Fully Convolutional Networks (FCN) and optical flow tracking. Our method exploits the ability of deep neural networks to produce accurate segmentations of highly deformable parts along with the high speed of optical flow. Furthermore, the pre-trained FCN can be fine-tuned on a small amount of medical images without the need to hand-craft features. We validated our method using existing and new benchmark datasets, covering both ex vivo and in vivo real clinical cases where different surgical instruments are employed. Two versions of the method are presented, non-real-time and real-time. The former, using only deep learning, achieves a balanced accuracy of 89.6% on a real clinical dataset, outperforming the (non-real-time) state of the art by 3.8% points. The latter, a combination of deep learning with optical flow tracking, yields an average balanced accuracy of 78.2% across all the validated datasets.
Deep Sequential Mosaicking of Fetoscopic Videos
Jul 15, 2019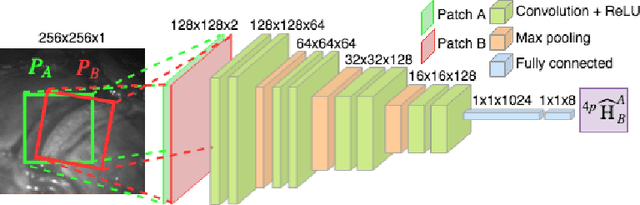
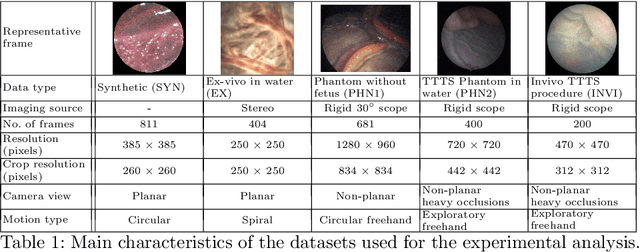
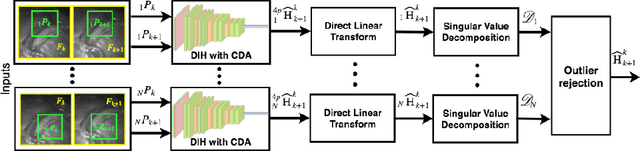
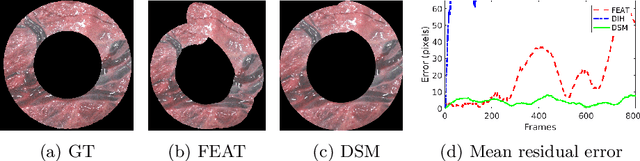
Abstract:Twin-to-twin transfusion syndrome treatment requires fetoscopic laser photocoagulation of placental vascular anastomoses to regulate blood flow to both fetuses. Limited field-of-view (FoV) and low visual quality during fetoscopy make it challenging to identify all vascular connections. Mosaicking can align multiple overlapping images to generate an image with increased FoV, however, existing techniques apply poorly to fetoscopy due to the low visual quality, texture paucity, and hence fail in longer sequences due to the drift accumulated over time. Deep learning techniques can facilitate in overcoming these challenges. Therefore, we present a new generalized Deep Sequential Mosaicking (DSM) framework for fetoscopic videos captured from different settings such as simulation, phantom, and real environments. DSM extends an existing deep image-based homography model to sequential data by proposing controlled data augmentation and outlier rejection methods. Unlike existing methods, DSM can handle visual variations due to specular highlights and reflection across adjacent frames, hence reducing the accumulated drift. We perform experimental validation and comparison using 5 diverse fetoscopic videos to demonstrate the robustness of our framework.
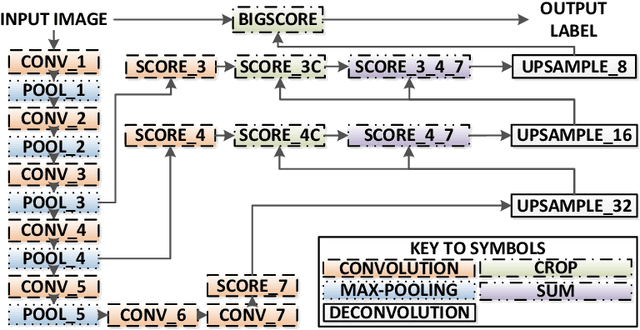
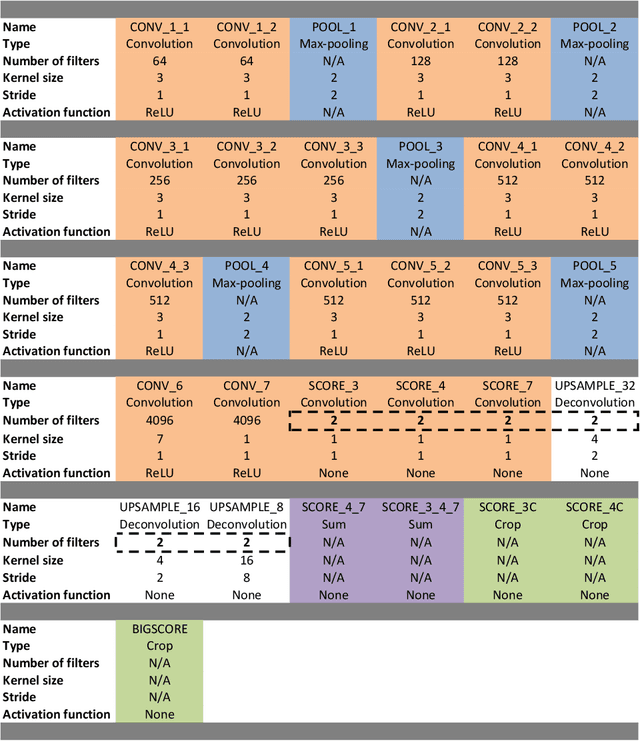
ToolNet: Holistically-Nested Real-Time Segmentation of Robotic Surgical Tools
Jul 04, 2017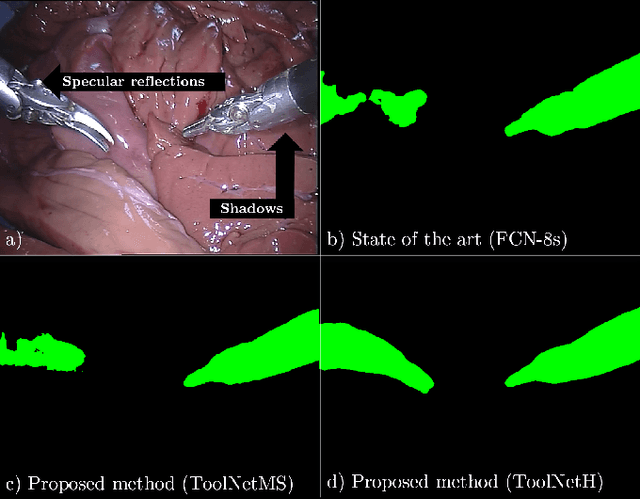
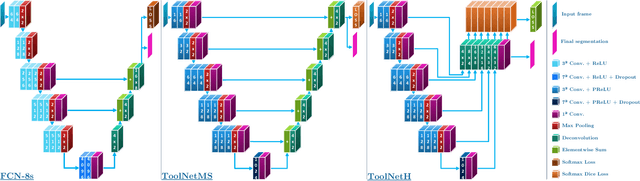
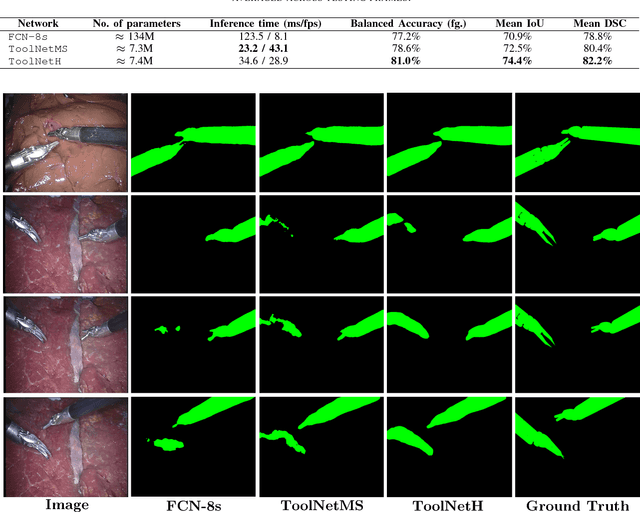
Abstract:Real-time tool segmentation from endoscopic videos is an essential part of many computer-assisted robotic surgical systems and of critical importance in robotic surgical data science. We propose two novel deep learning architectures for automatic segmentation of non-rigid surgical instruments. Both methods take advantage of automated deep-learning-based multi-scale feature extraction while trying to maintain an accurate segmentation quality at all resolutions. The two proposed methods encode the multi-scale constraint inside the network architecture. The first proposed architecture enforces it by cascaded aggregation of predictions and the second proposed network does it by means of a holistically-nested architecture where the loss at each scale is taken into account for the optimization process. As the proposed methods are for real-time semantic labeling, both present a reduced number of parameters. We propose the use of parametric rectified linear units for semantic labeling in these small architectures to increase the regularization ability of the design and maintain the segmentation accuracy without overfitting the training sets. We compare the proposed architectures against state-of-the-art fully convolutional networks. We validate our methods using existing benchmark datasets, including ex vivo cases with phantom tissue and different robotic surgical instruments present in the scene. Our results show a statistically significant improved Dice Similarity Coefficient over previous instrument segmentation methods. We analyze our design choices and discuss the key drivers for improving accuracy.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge