Ashish Rauniyar
Ensuring Trustworthy Medical Artificial Intelligence through Ethical and Philosophical Principles
Apr 29, 2023Abstract:Artificial intelligence (AI) methods have great potential to revolutionize numerous medical care by enhancing the experience of medical experts and patients. AI based computer-assisted diagnosis tools can have a tremendous benefit if they can outperform or perform similarly to the level of a clinical expert. As a result, advanced healthcare services can be affordable in developing nations, and the problem of a lack of expert medical practitioners can be addressed. AI based tools can save time, resources, and overall cost for patient treatment. Furthermore, in contrast to humans, AI can uncover complex relations in the data from a large set of inputs and even lead to new evidence-based knowledge in medicine. However, integrating AI in healthcare raises several ethical and philosophical concerns, such as bias, transparency, autonomy, responsibility and accountability, which must be addressed before integrating such tools into clinical settings. In this article, we emphasize recent advances in AI-assisted medical image analysis, existing standards, and the significance of comprehending ethical issues and best practices for the applications of AI in clinical settings. We cover the technical and ethical challenges of AI and the implications of deploying AI in hospitals and public organizations. We also discuss promising key measures and techniques to address the ethical challenges, data scarcity, racial bias, lack of transparency, and algorithmic bias. Finally, we provide our recommendation and future directions for addressing the ethical challenges associated with AI in healthcare applications, with the goal of deploying AI into the clinical settings to make the workflow more efficient, accurate, accessible, transparent, and reliable for the patient worldwide.
Federated Learning for Medical Applications: A Taxonomy, Current Trends, Challenges, and Future Research Directions
Aug 15, 2022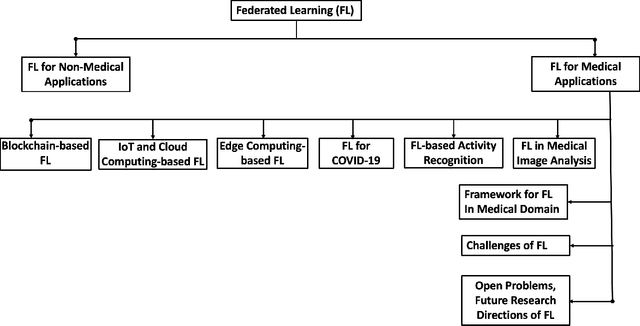
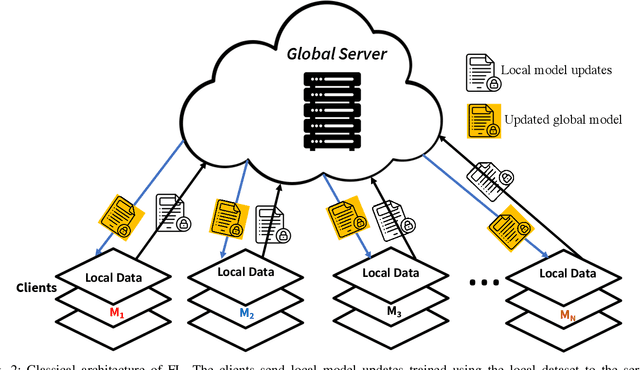
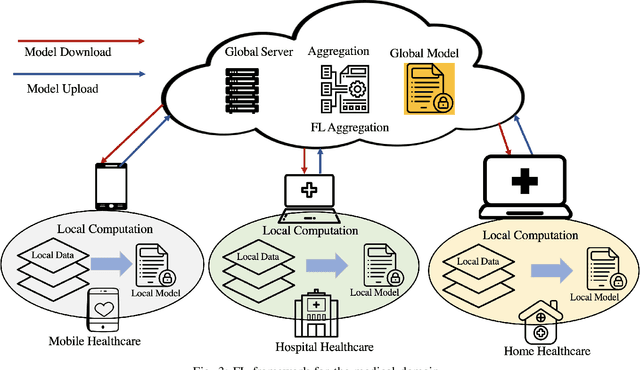
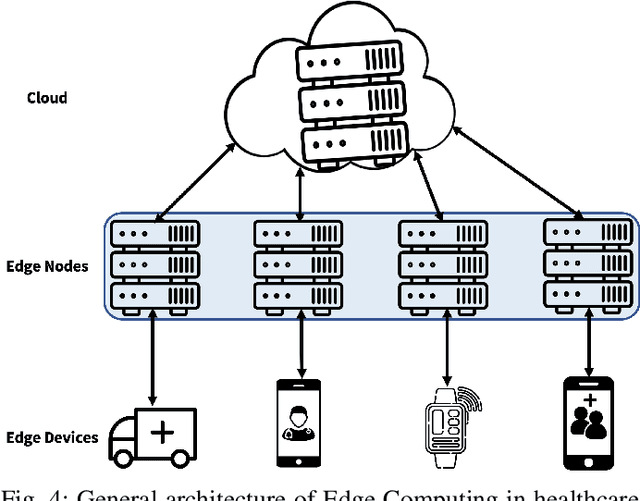
Abstract:With the advent of the IoT, AI, and ML/DL algorithms, the data-driven medical application has emerged as a promising tool for designing reliable and scalable diagnostic and prognostic models from medical data. This has attracted a great deal of attention from academia to industry in recent years. This has undoubtedly improved the quality of healthcare delivery. However, these AI-based medical applications still have poor adoption due to their difficulties in satisfying strict security, privacy, and quality of service standards (such as low latency). Moreover, medical data are usually fragmented and private, making it challenging to generate robust results across populations. Recent developments in federated learning (FL) have made it possible to train complex machine-learned models in a distributed manner. Thus, FL has become an active research domain, particularly processing the medical data at the edge of the network in a decentralized way to preserve privacy and security concerns. To this end, this survey paper highlights the current and future of FL technology in medical applications where data sharing is a significant burden. It also review and discuss the current research trends and their outcomes for designing reliable and scalable FL models. We outline the general FL's statistical problems, device challenges, security, privacy concerns, and its potential in the medical domain. Moreover, our study is also focused on medical applications where we highlight the burden of global cancer and the efficient use of FL for the development of computer-aided diagnosis tools for addressing them. We hope that this review serves as a checkpoint that sets forth the existing state-of-the-art works in a thorough manner and offers open problems and future research directions for this field.
Automatic Polyp Segmentation using U-Net-ResNet50
Dec 30, 2020
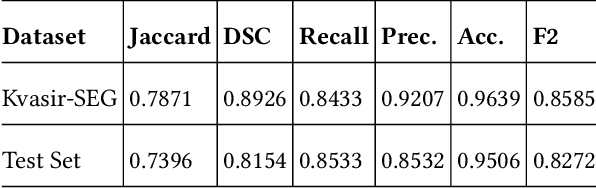
Abstract:Polyps are the predecessors to colorectal cancer which is considered as one of the leading causes of cancer-related deaths worldwide. Colonoscopy is the standard procedure for the identification, localization, and removal of colorectal polyps. Due to variability in shape, size, and surrounding tissue similarity, colorectal polyps are often missed by the clinicians during colonoscopy. With the use of an automatic, accurate, and fast polyp segmentation method during the colonoscopy, many colorectal polyps can be easily detected and removed. The ``Medico automatic polyp segmentation challenge'' provides an opportunity to study polyp segmentation and build an efficient and accurate segmentation algorithm. We use the U-Net with pre-trained ResNet50 as the encoder for the polyp segmentation. The model is trained on Kvasir-SEG dataset provided for the challenge and tested on the organizer's dataset and achieves a dice coefficient of 0.8154, Jaccard of 0.7396, recall of 0.8533, precision of 0.8532, accuracy of 0.9506, and F2 score of 0.8272, demonstrating the generalization ability of our model.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge