Few-Shot Learning for Annotation-Efficient Nucleus Instance Segmentation
Paper and Code
Feb 28, 2024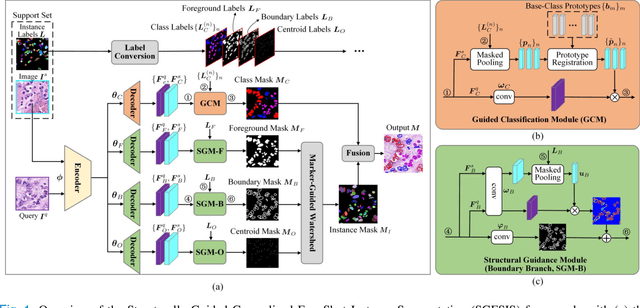
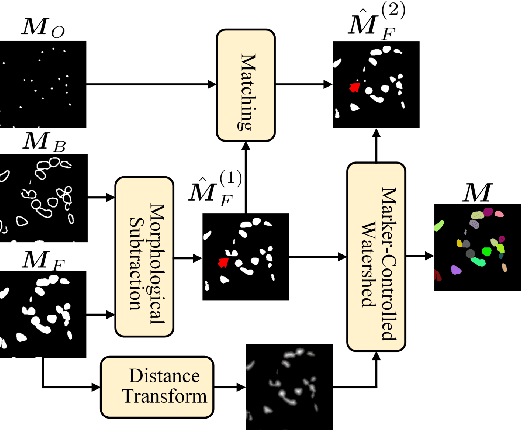
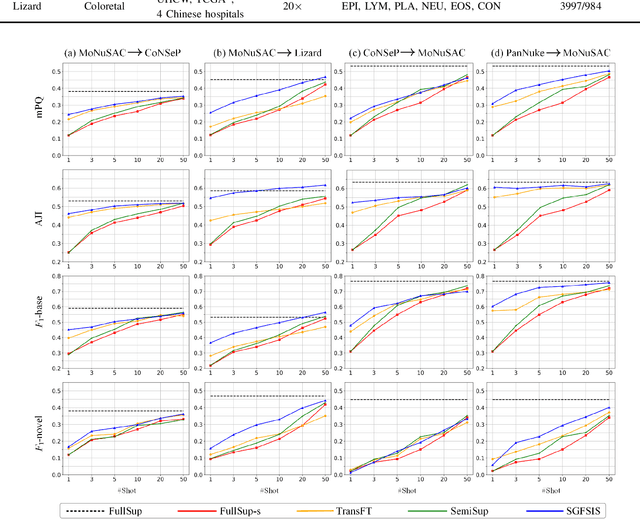
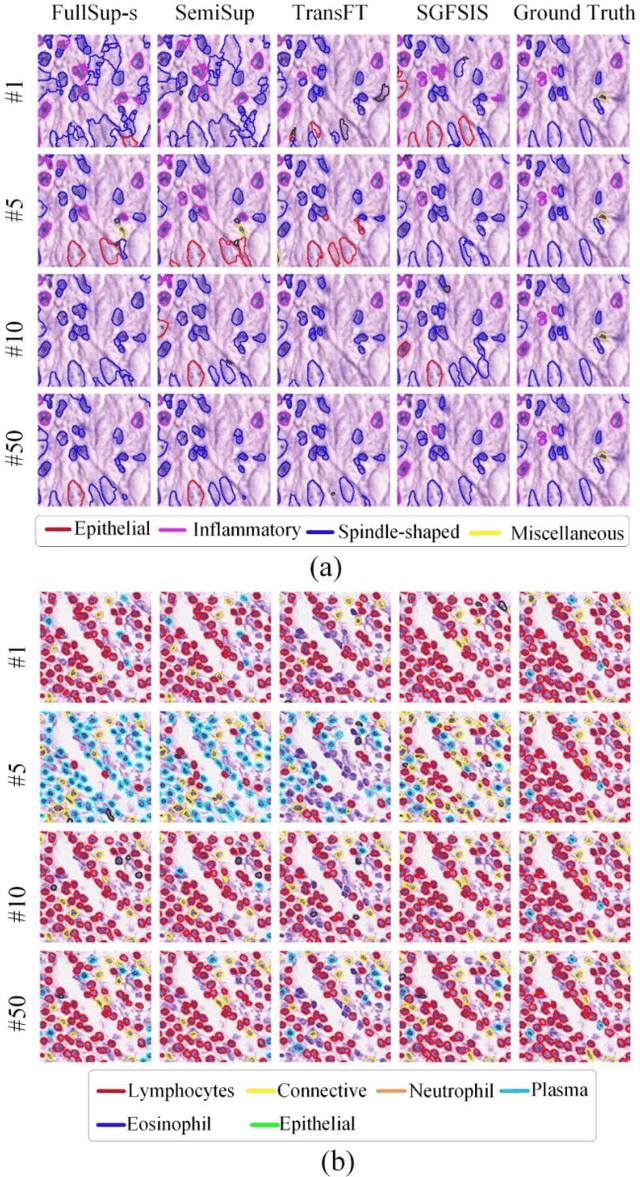
Nucleus instance segmentation from histopathology images suffers from the extremely laborious and expert-dependent annotation of nucleus instances. As a promising solution to this task, annotation-efficient deep learning paradigms have recently attracted much research interest, such as weakly-/semi-supervised learning, generative adversarial learning, etc. In this paper, we propose to formulate annotation-efficient nucleus instance segmentation from the perspective of few-shot learning (FSL). Our work was motivated by that, with the prosperity of computational pathology, an increasing number of fully-annotated datasets are publicly accessible, and we hope to leverage these external datasets to assist nucleus instance segmentation on the target dataset which only has very limited annotation. To achieve this goal, we adopt the meta-learning based FSL paradigm, which however has to be tailored in two substantial aspects before adapting to our task. First, since the novel classes may be inconsistent with those of the external dataset, we extend the basic definition of few-shot instance segmentation (FSIS) to generalized few-shot instance segmentation (GFSIS). Second, to cope with the intrinsic challenges of nucleus segmentation, including touching between adjacent cells, cellular heterogeneity, etc., we further introduce a structural guidance mechanism into the GFSIS network, finally leading to a unified Structurally-Guided Generalized Few-Shot Instance Segmentation (SGFSIS) framework. Extensive experiments on a couple of publicly accessible datasets demonstrate that, SGFSIS can outperform other annotation-efficient learning baselines, including semi-supervised learning, simple transfer learning, etc., with comparable performance to fully supervised learning with less than 5% annotations.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge