Ziyun Liang
IterMask3D: Unsupervised Anomaly Detection and Segmentation with Test-Time Iterative Mask Refinement in 3D Brain MR
Apr 07, 2025Abstract:Unsupervised anomaly detection and segmentation methods train a model to learn the training distribution as 'normal'. In the testing phase, they identify patterns that deviate from this normal distribution as 'anomalies'. To learn the `normal' distribution, prevailing methods corrupt the images and train a model to reconstruct them. During testing, the model attempts to reconstruct corrupted inputs based on the learned 'normal' distribution. Deviations from this distribution lead to high reconstruction errors, which indicate potential anomalies. However, corrupting an input image inevitably causes information loss even in normal regions, leading to suboptimal reconstruction and an increased risk of false positives. To alleviate this, we propose IterMask3D, an iterative spatial mask-refining strategy designed for 3D brain MRI. We iteratively spatially mask areas of the image as corruption and reconstruct them, then shrink the mask based on reconstruction error. This process iteratively unmasks 'normal' areas to the model, whose information further guides reconstruction of 'normal' patterns under the mask to be reconstructed accurately, reducing false positives. In addition, to achieve better reconstruction performance, we also propose using high-frequency image content as additional structural information to guide the reconstruction of the masked area. Extensive experiments on the detection of both synthetic and real-world imaging artifacts, as well as segmentation of various pathological lesions across multiple MRI sequences, consistently demonstrate the effectiveness of our proposed method.
Continuous Online Adaptation Driven by User Interaction for Medical Image Segmentation
Mar 09, 2025Abstract:Interactive segmentation models use real-time user interactions, such as mouse clicks, as extra inputs to dynamically refine the model predictions. After model deployment, user corrections of model predictions could be used to adapt the model to the post-deployment data distribution, countering distribution-shift and enhancing reliability. Motivated by this, we introduce an online adaptation framework that enables an interactive segmentation model to continuously learn from user interaction and improve its performance on new data distributions, as it processes a sequence of test images. We introduce the Gaussian Point Loss function to train the model how to leverage user clicks, along with a two-stage online optimization method that adapts the model using the corrected predictions generated via user interactions. We demonstrate that this simple and therefore practical approach is very effective. Experiments on 5 fundus and 4 brain MRI databases demonstrate that our method outperforms existing approaches under various data distribution shifts, including segmentation of image modalities and pathologies not seen during training.
Feasibility of Federated Learning from Client Databases with Different Brain Diseases and MRI Modalities
Jun 17, 2024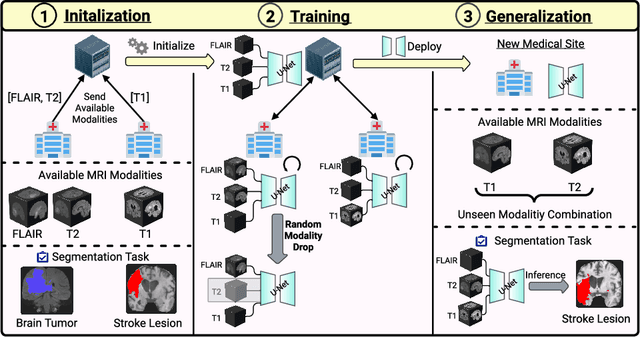
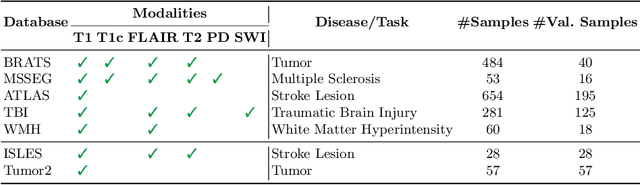
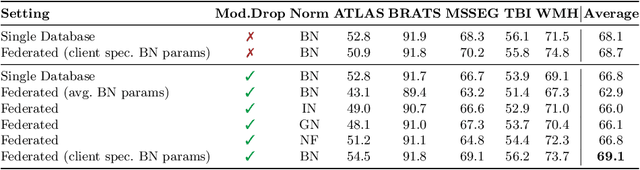
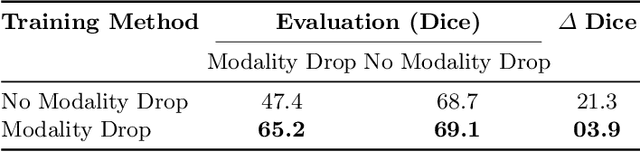
Abstract:Segmentation models for brain lesions in MRI are commonly developed for a specific disease and trained on data with a predefined set of MRI modalities. Each such model cannot segment the disease using data with a different set of MRI modalities, nor can it segment any other type of disease. Moreover, this training paradigm does not allow a model to benefit from learning from heterogeneous databases that may contain scans and segmentation labels for different types of brain pathologies and diverse sets of MRI modalities. Is it feasible to use Federated Learning (FL) for training a single model on client databases that contain scans and labels of different brain pathologies and diverse sets of MRI modalities? We demonstrate promising results by combining appropriate, simple, and practical modifications to the model and training strategy: Designing a model with input channels that cover the whole set of modalities available across clients, training with random modality drop, and exploring the effects of feature normalization methods. Evaluation on 7 brain MRI databases with 5 different diseases shows that such FL framework can train a single model that is shown to be very promising in segmenting all disease types seen during training. Importantly, it is able to segment these diseases in new databases that contain sets of modalities different from those in training clients. These results demonstrate, for the first time, feasibility and effectiveness of using FL to train a single segmentation model on decentralised data with diverse brain diseases and MRI modalities, a necessary step towards leveraging heterogeneous real-world databases. Code will be made available at: https://github.com/FelixWag/FL-MultiDisease-MRI
IterMask2: Iterative Unsupervised Anomaly Segmentation via Spatial and Frequency Masking for Brain Lesions in MRI
Jun 04, 2024Abstract:Unsupervised anomaly segmentation approaches to pathology segmentation train a model on images of healthy subjects, that they define as the 'normal' data distribution. At inference, they aim to segment any pathologies in new images as 'anomalies', as they exhibit patterns that deviate from those in 'normal' training data. Prevailing methods follow the 'corrupt-and-reconstruct' paradigm. They intentionally corrupt an input image, reconstruct it to follow the learned 'normal' distribution, and subsequently segment anomalies based on reconstruction error. Corrupting an input image, however, inevitably leads to suboptimal reconstruction even of normal regions, causing false positives. To alleviate this, we propose a novel iterative spatial mask-refining strategy IterMask2. We iteratively mask areas of the image, reconstruct them, and update the mask based on reconstruction error. This iterative process progressively adds information about areas that are confidently normal as per the model. The increasing content guides reconstruction of nearby masked areas, improving reconstruction of normal tissue under these areas, reducing false positives. We also use high-frequency image content as an auxiliary input to provide additional structural information for masked areas. This further improves reconstruction error of normal in comparison to anomalous areas, facilitating segmentation of the latter. We conduct experiments on several brain lesion datasets and demonstrate effectiveness of our method. Code is available at: https://github.com/ZiyunLiang/IterMasks2
Feasibility and benefits of joint learning from MRI databases with different brain diseases and modalities for segmentation
May 28, 2024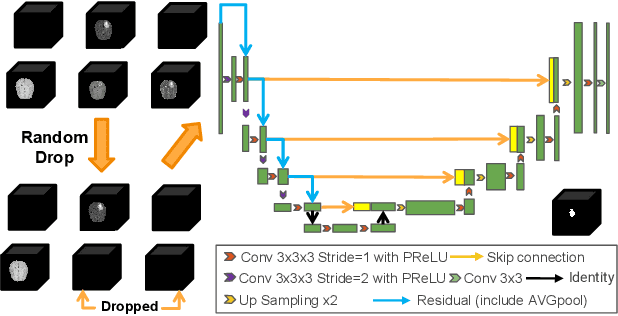
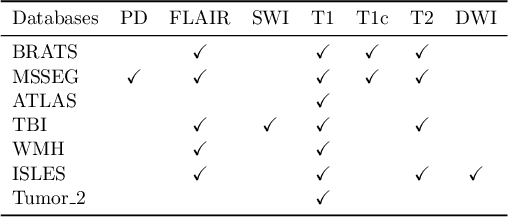
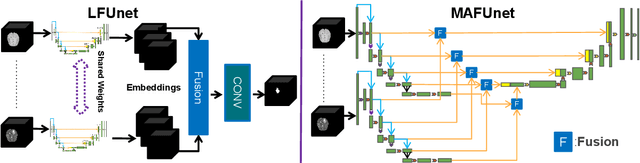

Abstract:Models for segmentation of brain lesions in multi-modal MRI are commonly trained for a specific pathology using a single database with a predefined set of MRI modalities, determined by a protocol for the specific disease. This work explores the following open questions: Is it feasible to train a model using multiple databases that contain varying sets of MRI modalities and annotations for different brain pathologies? Will this joint learning benefit performance on the sets of modalities and pathologies available during training? Will it enable analysis of new databases with different sets of modalities and pathologies? We develop and compare different methods and show that promising results can be achieved with appropriate, simple and practical alterations to the model and training framework. We experiment with 7 databases containing 5 types of brain pathologies and different sets of MRI modalities. Results demonstrate, for the first time, that joint training on multi-modal MRI databases with different brain pathologies and sets of modalities is feasible and offers practical benefits. It enables a single model to segment pathologies encountered during training in diverse sets of modalities, while facilitating segmentation of new types of pathologies such as via follow-up fine-tuning. The insights this study provides into the potential and limitations of this paradigm should prove useful for guiding future advances in the direction. Code and pretrained models: https://github.com/WenTXuL/MultiUnet
* Accepted to MIDL 2024
Modality Cycles with Masked Conditional Diffusion for Unsupervised Anomaly Segmentation in MRI
Aug 30, 2023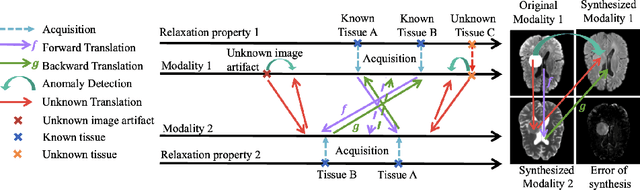
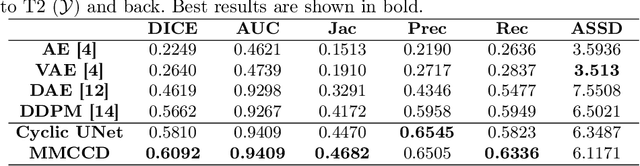
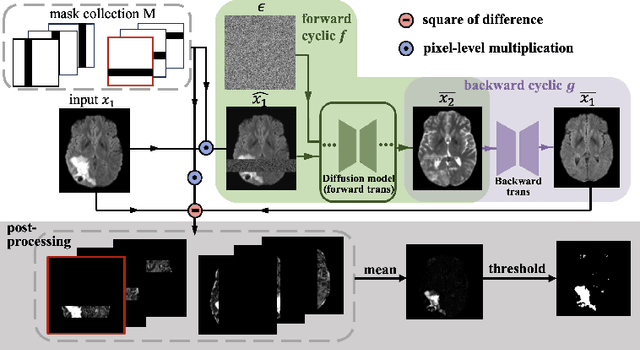
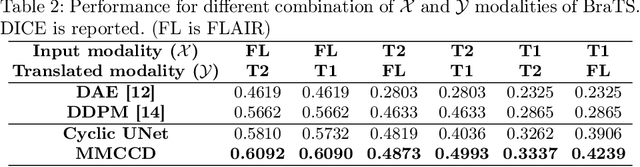
Abstract:Unsupervised anomaly segmentation aims to detect patterns that are distinct from any patterns processed during training, commonly called abnormal or out-of-distribution patterns, without providing any associated manual segmentations. Since anomalies during deployment can lead to model failure, detecting the anomaly can enhance the reliability of models, which is valuable in high-risk domains like medical imaging. This paper introduces Masked Modality Cycles with Conditional Diffusion (MMCCD), a method that enables segmentation of anomalies across diverse patterns in multimodal MRI. The method is based on two fundamental ideas. First, we propose the use of cyclic modality translation as a mechanism for enabling abnormality detection. Image-translation models learn tissue-specific modality mappings, which are characteristic of tissue physiology. Thus, these learned mappings fail to translate tissues or image patterns that have never been encountered during training, and the error enables their segmentation. Furthermore, we combine image translation with a masked conditional diffusion model, which attempts to `imagine' what tissue exists under a masked area, further exposing unknown patterns as the generative model fails to recreate them. We evaluate our method on a proxy task by training on healthy-looking slices of BraTS2021 multi-modality MRIs and testing on slices with tumors. We show that our method compares favorably to previous unsupervised approaches based on image reconstruction and denoising with autoencoders and diffusion models.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge