Yashaswi Pathak
Maximum Reward Formulation In Reinforcement Learning
Oct 08, 2020
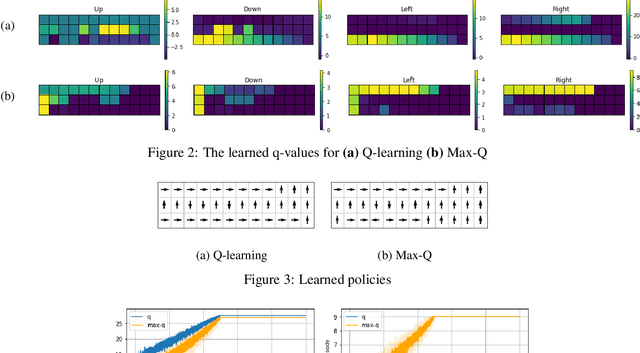
Abstract:Reinforcement learning (RL) algorithms typically deal with maximizing the expected cumulative return (discounted or undiscounted, finite or infinite horizon). However, several crucial applications in the real world, such as drug discovery, do not fit within this framework because an RL agent only needs to identify states (molecules) that achieve the highest reward within a trajectory and does not need to optimize for the expected cumulative return. In this work, we formulate an objective function to maximize the expected maximum reward along a trajectory, derive a novel functional form of the Bellman equation, introduce the corresponding Bellman operators, and provide a proof of convergence. Using this formulation, we achieve state-of-the-art results on the task of molecule generation that mimics a real-world drug discovery pipeline.
Learning To Navigate The Synthetically Accessible Chemical Space Using Reinforcement Learning
May 20, 2020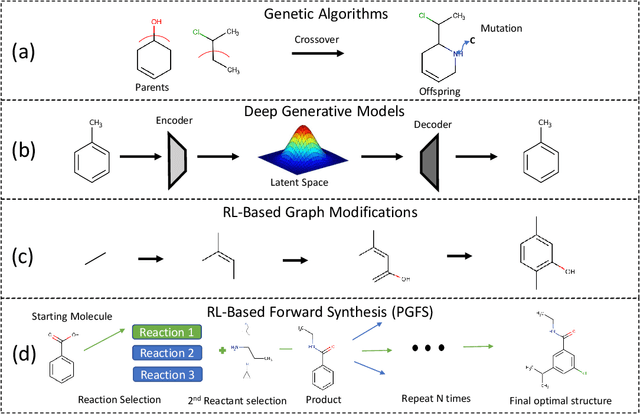
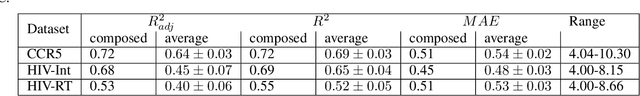

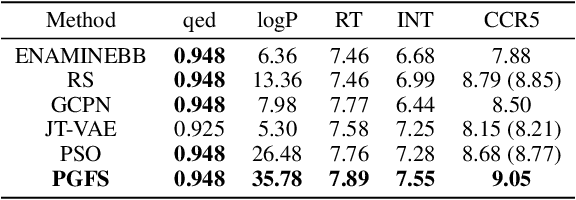
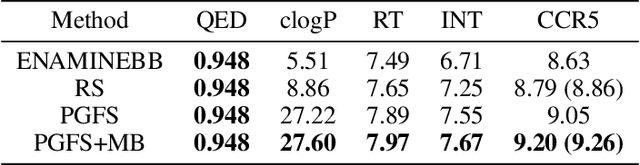
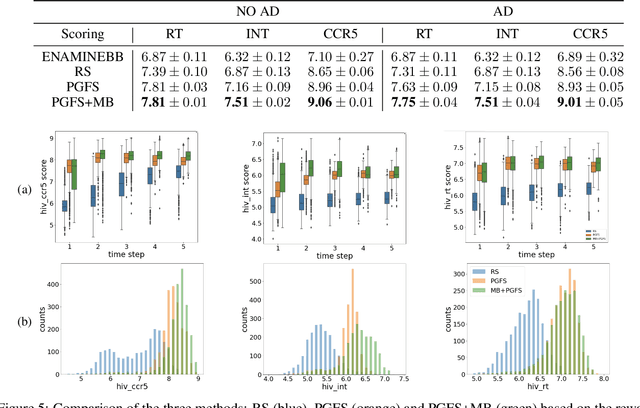
Abstract:Over the last decade, there has been significant progress in the field of machine learning for de novo drug design, particularly in deep generative models. However, current generative approaches exhibit a significant challenge as they do not ensure that the proposed molecular structures can be feasibly synthesized nor do they provide the synthesis routes of the proposed small molecules, thereby seriously limiting their practical applicability. In this work, we propose a novel forward synthesis framework powered by reinforcement learning (RL) for de novo drug design, Policy Gradient for Forward Synthesis (PGFS), that addresses this challenge by embedding the concept of synthetic accessibility directly into the de novo drug design system. In this setup, the agent learns to navigate through the immense synthetically accessible chemical space by subjecting commercially available small molecule building blocks to valid chemical reactions at every time step of the iterative virtual multi-step synthesis process. The proposed environment for drug discovery provides a highly challenging test-bed for RL algorithms owing to the large state space and high-dimensional continuous action space with hierarchical actions. PGFS achieves state-of-the-art performance in generating structures with high QED and penalized clogP. Moreover, we validate PGFS in an in-silico proof-of-concept associated with three HIV targets. Finally, we describe how the end-to-end training conceptualized in this study represents an important paradigm in radically expanding the synthesizable chemical space and automating the drug discovery process.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge