Yanyi Chu
Latent Diffusion Models for Controllable RNA Sequence Generation
Sep 15, 2024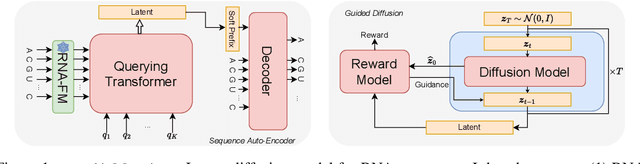
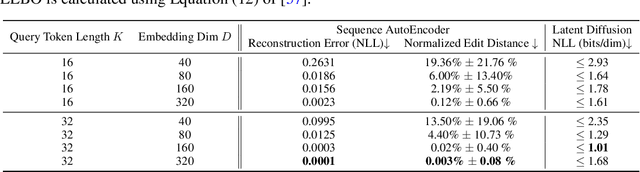

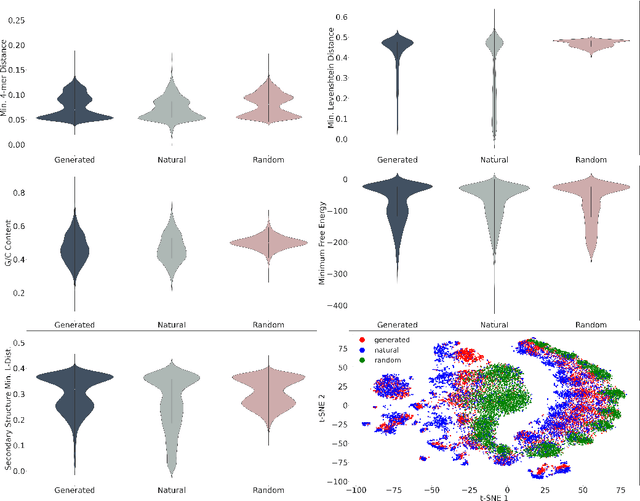
Abstract:This paper presents RNAdiffusion, a latent diffusion model for generating and optimizing discrete RNA sequences. RNA is a particularly dynamic and versatile molecule in biological processes. RNA sequences exhibit high variability and diversity, characterized by their variable lengths, flexible three-dimensional structures, and diverse functions. We utilize pretrained BERT-type models to encode raw RNAs into token-level biologically meaningful representations. A Q-Former is employed to compress these representations into a fixed-length set of latent vectors, with an autoregressive decoder trained to reconstruct RNA sequences from these latent variables. We then develop a continuous diffusion model within this latent space. To enable optimization, we train reward networks to estimate functional properties of RNA from the latent variables. We employ gradient-based guidance during the backward diffusion process, aiming to generate RNA sequences that are optimized for higher rewards. Empirical experiments confirm that RNAdiffusion generates non-coding RNAs that align with natural distributions across various biological indicators. We fine-tuned the diffusion model on untranslated regions (UTRs) of mRNA and optimize sample sequences for protein translation efficiencies. Our guided diffusion model effectively generates diverse UTR sequences with high Mean Ribosome Loading (MRL) and Translation Efficiency (TE), surpassing baselines. These results hold promise for studies on RNA sequence-function relationships, protein synthesis, and enhancing therapeutic RNA design.
A 5' UTR Language Model for Decoding Untranslated Regions of mRNA and Function Predictions
Oct 06, 2023Abstract:The 5' UTR, a regulatory region at the beginning of an mRNA molecule, plays a crucial role in regulating the translation process and impacts the protein expression level. Language models have showcased their effectiveness in decoding the functions of protein and genome sequences. Here, we introduced a language model for 5' UTR, which we refer to as the UTR-LM. The UTR-LM is pre-trained on endogenous 5' UTRs from multiple species and is further augmented with supervised information including secondary structure and minimum free energy. We fine-tuned the UTR-LM in a variety of downstream tasks. The model outperformed the best-known benchmark by up to 42% for predicting the Mean Ribosome Loading, and by up to 60% for predicting the Translation Efficiency and the mRNA Expression Level. The model also applies to identifying unannotated Internal Ribosome Entry Sites within the untranslated region and improves the AUPR from 0.37 to 0.52 compared to the best baseline. Further, we designed a library of 211 novel 5' UTRs with high predicted values of translation efficiency and evaluated them via a wet-lab assay. Experiment results confirmed that our top designs achieved a 32.5% increase in protein production level relative to well-established 5' UTR optimized for therapeutics.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge