Won Young Tak
Towards Efficient and Accurate CT Segmentation via Edge-Preserving Probabilistic Downsampling
Apr 05, 2024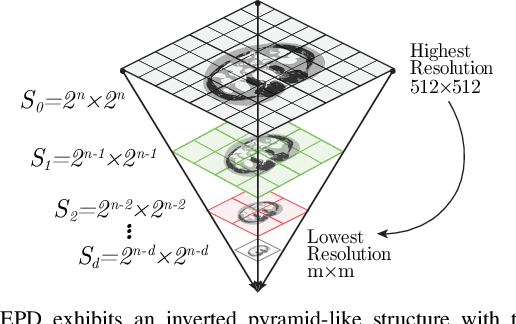

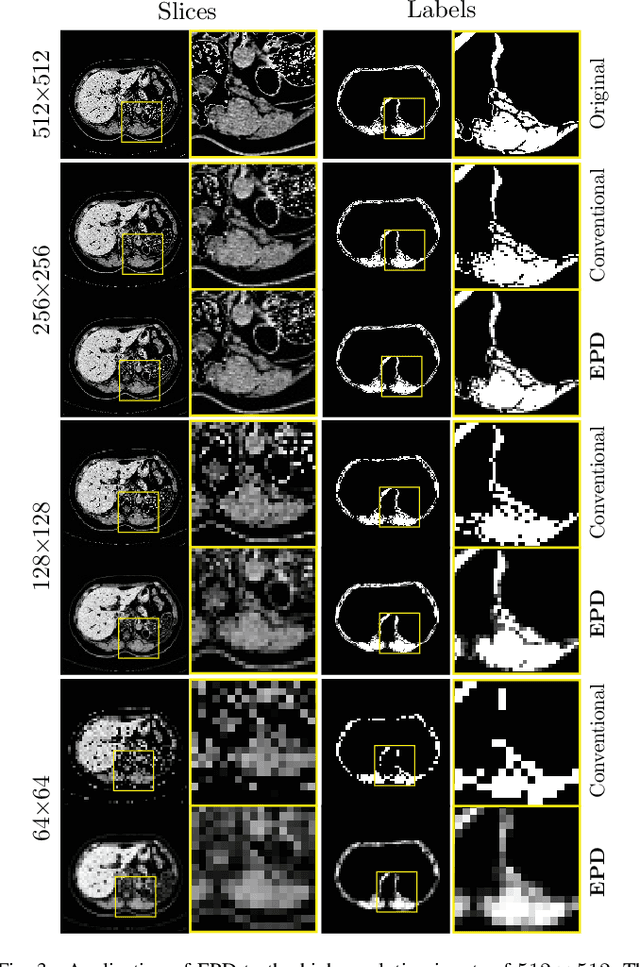
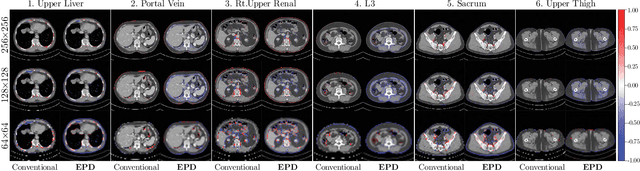
Abstract:Downsampling images and labels, often necessitated by limited resources or to expedite network training, leads to the loss of small objects and thin boundaries. This undermines the segmentation network's capacity to interpret images accurately and predict detailed labels, resulting in diminished performance compared to processing at original resolutions. This situation exemplifies the trade-off between efficiency and accuracy, with higher downsampling factors further impairing segmentation outcomes. Preserving information during downsampling is especially critical for medical image segmentation tasks. To tackle this challenge, we introduce a novel method named Edge-preserving Probabilistic Downsampling (EPD). It utilizes class uncertainty within a local window to produce soft labels, with the window size dictating the downsampling factor. This enables a network to produce quality predictions at low resolutions. Beyond preserving edge details more effectively than conventional nearest-neighbor downsampling, employing a similar algorithm for images, it surpasses bilinear interpolation in image downsampling, enhancing overall performance. Our method significantly improved Intersection over Union (IoU) to 2.85%, 8.65%, and 11.89% when downsampling data to 1/2, 1/4, and 1/8, respectively, compared to conventional interpolation methods.
Deep Metric Learning-based Image Retrieval System for Chest Radiograph and its Clinical Applications in COVID-19
Nov 26, 2020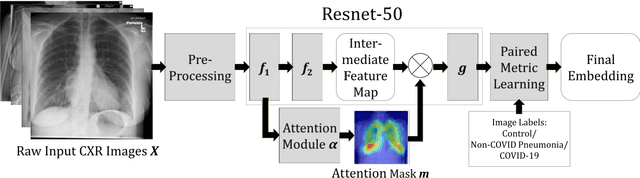
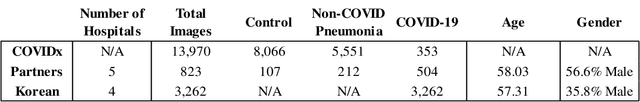
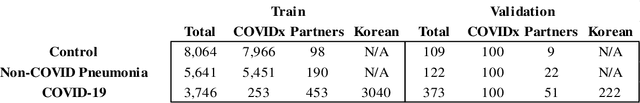
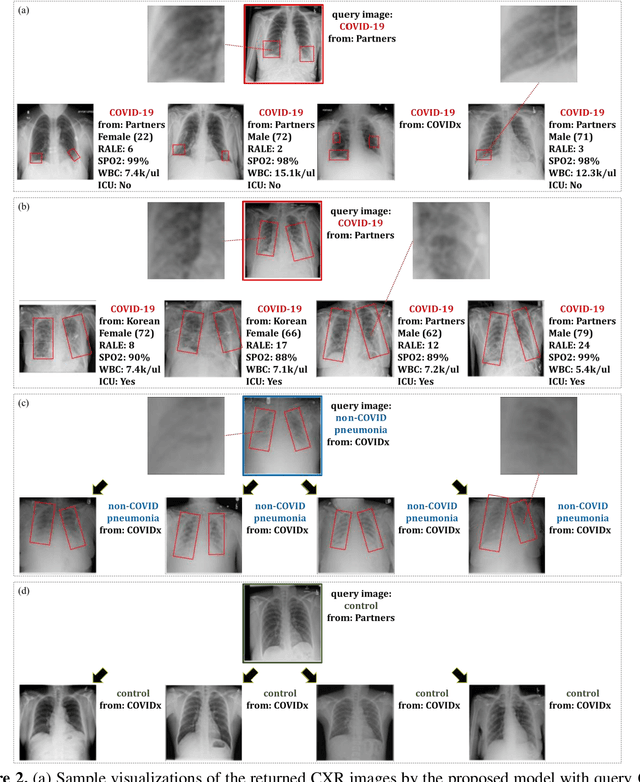
Abstract:In recent years, deep learning-based image analysis methods have been widely applied in computer-aided detection, diagnosis and prognosis, and has shown its value during the public health crisis of the novel coronavirus disease 2019 (COVID-19) pandemic. Chest radiograph (CXR) has been playing a crucial role in COVID-19 patient triaging, diagnosing and monitoring, particularly in the United States. Considering the mixed and unspecific signals in CXR, an image retrieval model of CXR that provides both similar images and associated clinical information can be more clinically meaningful than a direct image diagnostic model. In this work we develop a novel CXR image retrieval model based on deep metric learning. Unlike traditional diagnostic models which aims at learning the direct mapping from images to labels, the proposed model aims at learning the optimized embedding space of images, where images with the same labels and similar contents are pulled together. It utilizes multi-similarity loss with hard-mining sampling strategy and attention mechanism to learn the optimized embedding space, and provides similar images to the query image. The model is trained and validated on an international multi-site COVID-19 dataset collected from 3 different sources. Experimental results of COVID-19 image retrieval and diagnosis tasks show that the proposed model can serve as a robust solution for CXR analysis and patient management for COVID-19. The model is also tested on its transferability on a different clinical decision support task, where the pre-trained model is applied to extract image features from a new dataset without any further training. These results demonstrate our deep metric learning based image retrieval model is highly efficient in the CXR retrieval, diagnosis and prognosis, and thus has great clinical value for the treatment and management of COVID-19 patients.
Deep Learning-based Four-region Lung Segmentation in Chest Radiography for COVID-19 Diagnosis
Sep 26, 2020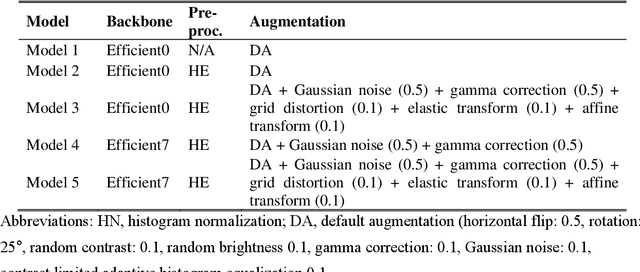
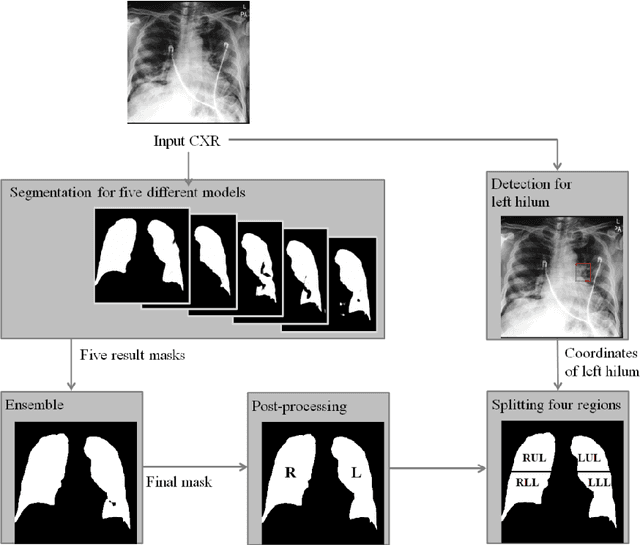
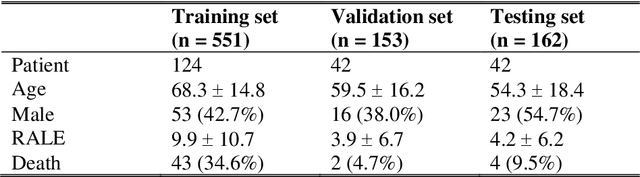
Abstract:Purpose. Imaging plays an important role in assessing severity of COVID 19 pneumonia. However, semantic interpretation of chest radiography (CXR) findings does not include quantitative description of radiographic opacities. Most current AI assisted CXR image analysis framework do not quantify for regional variations of disease. To address these, we proposed a four region lung segmentation method to assist accurate quantification of COVID 19 pneumonia. Methods. A segmentation model to separate left and right lung is firstly applied, and then a carina and left hilum detection network is used, which are the clinical landmarks to separate the upper and lower lungs. To improve the segmentation performance of COVID 19 images, ensemble strategy incorporating five models is exploited. Using each region, we evaluated the clinical relevance of the proposed method with the Radiographic Assessment of the Quality of Lung Edema (RALE). Results. The proposed ensemble strategy showed dice score of 0.900, which is significantly higher than conventional methods (0.854 0.889). Mean intensities of segmented four regions indicate positive correlation to the extent and density scores of pulmonary opacities under the RALE framework. Conclusion. A deep learning based model in CXR can accurately segment and quantify regional distribution of pulmonary opacities in patients with COVID 19 pneumonia.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge