Wajid Arshad Abbasi
Investigating Knowledge Distillation Through Neural Networks for Protein Binding Affinity Prediction
Jan 07, 2026Abstract:The trade-off between predictive accuracy and data availability makes it difficult to predict protein--protein binding affinity accurately. The lack of experimentally resolved protein structures limits the performance of structure-based machine learning models, which generally outperform sequence-based methods. In order to overcome this constraint, we suggest a regression framework based on knowledge distillation that uses protein structural data during training and only needs sequence data during inference. The suggested method uses binding affinity labels and intermediate feature representations to jointly supervise the training of a sequence-based student network under the guidance of a structure-informed teacher network. Leave-One-Complex-Out (LOCO) cross-validation was used to assess the framework on a non-redundant protein--protein binding affinity benchmark dataset. A maximum Pearson correlation coefficient (P_r) of 0.375 and an RMSE of 2.712 kcal/mol were obtained by sequence-only baseline models, whereas a P_r of 0.512 and an RMSE of 2.445 kcal/mol were obtained by structure-based models. With a P_r of 0.481 and an RMSE of 2.488 kcal/mol, the distillation-based student model greatly enhanced sequence-only performance. Improved agreement and decreased bias were further confirmed by thorough error analyses. With the potential to close the performance gap between sequence-based and structure-based models as larger datasets become available, these findings show that knowledge distillation is an efficient method for transferring structural knowledge to sequence-based predictors. The source code for running inference with the proposed distillation-based binding affinity predictor can be accessed at https://github.com/wajidarshad/ProteinAffinityKD.
COVIDX: Computer-aided diagnosis of Covid-19 and its severity prediction with raw digital chest X-ray images
Dec 25, 2020
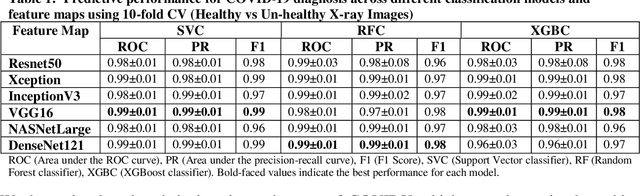

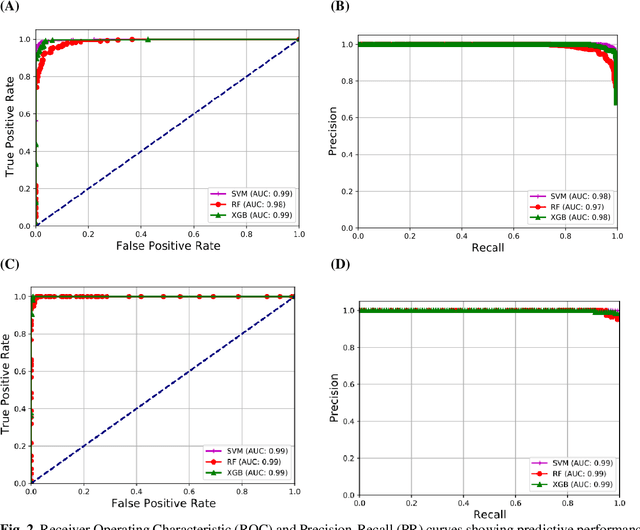
Abstract:Coronavirus disease (COVID-19) is a contagious infection caused by severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) and it has infected and killed millions of people across the globe. In the absence of specific drugs or vaccines for the treatment of COVID-19 and the limitation of prevailing diagnostic techniques, there is a requirement for some alternate automatic screening systems that can be used by the physicians to quickly identify and isolate the infected patients. A chest X-ray (CXR) image can be used as an alternative modality to detect and diagnose the COVID-19. In this study, we present an automatic COVID-19 diagnostic and severity prediction (COVIDX) system that uses deep feature maps from CXR images to diagnose COVID-19 and its severity prediction. The proposed system uses a three-phase classification approach (healthy vs unhealthy, COVID-19 vs Pneumonia, and COVID-19 severity) using different shallow supervised classification algorithms. We evaluated COVIDX not only through 10-fold cross2 validation and by using an external validation dataset but also in real settings by involving an experienced radiologist. In all the evaluation settings, COVIDX outperforms all the existing stateof-the-art methods designed for this purpose. We made COVIDX easily accessible through a cloud-based webserver and python code available at https://sites.google.com/view/wajidarshad/software and https://github.com/wajidarshad/covidx, respectively.
PANDA: Predicting the change in proteins binding affinity upon mutations using sequence information
Sep 16, 2020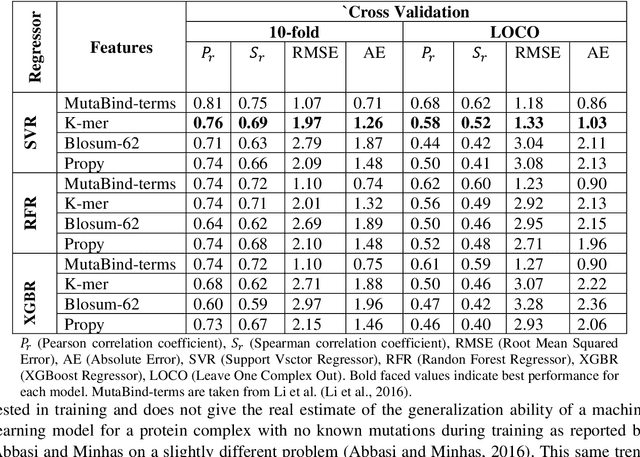

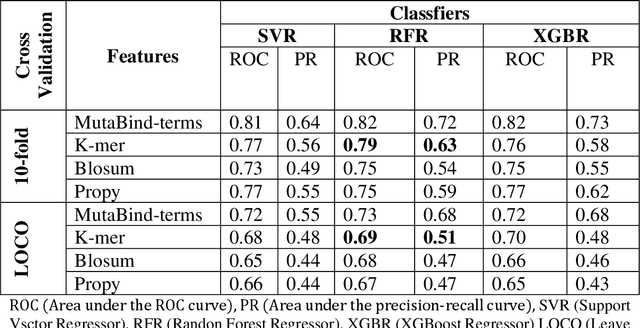
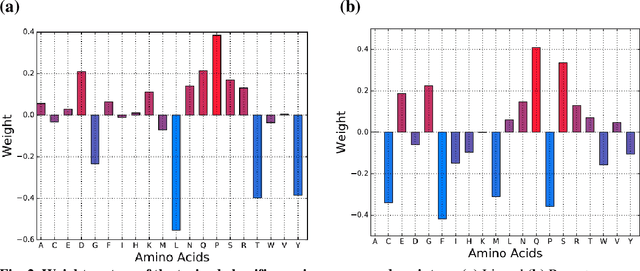
Abstract:Accurately determining a change in protein binding affinity upon mutations is important for the discovery and design of novel therapeutics and to assist mutagenesis studies. Determination of change in binding affinity upon mutations requires sophisticated, expensive, and time-consuming wet-lab experiments that can be aided with computational methods. Most of the computational prediction techniques require protein structures that limit their applicability to protein complexes with known structures. In this work, we explore the sequence-based prediction of change in protein binding affinity upon mutation. We have used protein sequence information instead of protein structures along with machine learning techniques to accurately predict the change in protein binding affinity upon mutation. Our proposed sequence-based novel change in protein binding affinity predictor called PANDA gives better accuracy than existing methods over the same validation set as well as on an external independent test dataset. On an external test dataset, our proposed method gives a maximum Pearson correlation coefficient of 0.52 in comparison to the state-of-the-art existing protein structure-based method called MutaBind which gives a maximum Pearson correlation coefficient of 0.59. Our proposed protein sequence-based method, to predict a change in binding affinity upon mutations, has wide applicability and comparable performance in comparison to existing protein structure-based methods. A cloud-based webserver implementation of PANDA and its python code is available at https://sites.google.com/view/wajidarshad/software and https://github.com/wajidarshad/panda.
ISLAND: In-Silico Prediction of Proteins Binding Affinity Using Sequence Descriptors
Mar 22, 2018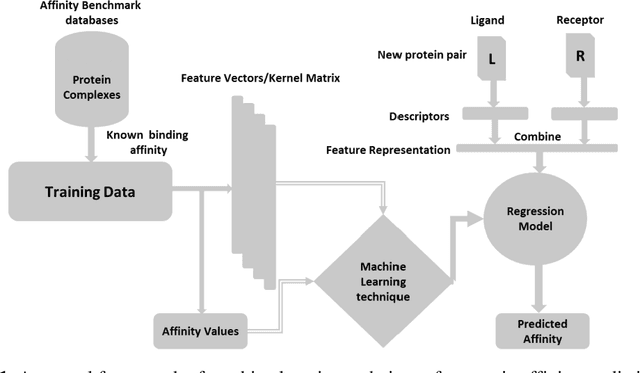
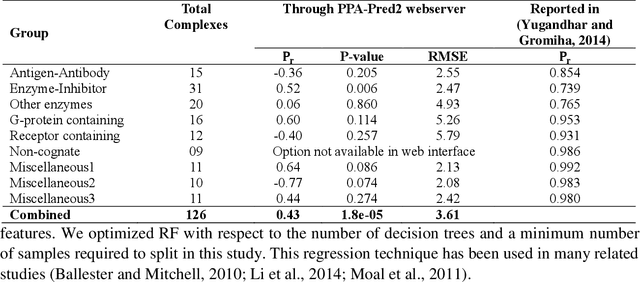
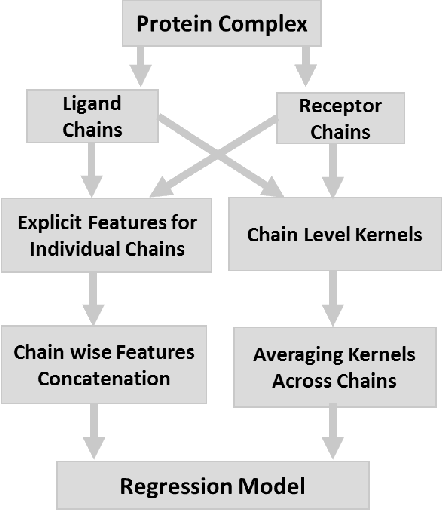
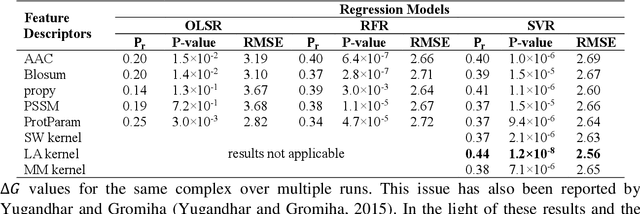
Abstract:Determination of binding affinity of proteins in the formation of protein complexes requires sophisticated, expensive and time-consuming experimentation which can be replaced with computational methods. Most computational prediction techniques require protein structures which limit their applicability to protein complexes with known structures. In this work, we explore sequence based protein binding affinity prediction using machine learning. Our paper highlights the fact that the generalization performance of even the state of the art sequence-only predictor of binding affinity is far from satisfactory and that the development of effective and practical methods in this domain is still an open problem. We also propose a novel sequence-only predictor of binding affinity called ISLAND which gives better accuracy than existing methods over the same validation set as well as on external independent test dataset. A cloud-based webserver implementation of ISLAND and its Python code are available at the URL: http://faculty.pieas.edu.pk/fayyaz/software.html#island.
Training large margin host-pathogen protein-protein interaction predictors
Nov 21, 2017



Abstract:Detection of protein-protein interactions (PPIs) plays a vital role in molecular biology. Particularly, infections are caused by the interactions of host and pathogen proteins. It is important to identify host-pathogen interactions (HPIs) to discover new drugs to counter infectious diseases. Conventional wet lab PPI prediction techniques have limitations in terms of large scale application and budget. Hence, computational approaches are developed to predict PPIs. This study aims to develop large margin machine learning models to predict interspecies PPIs with a special interest in host-pathogen protein interactions (HPIs). Especially, we focus on seeking answers to three queries that arise while developing an HPI predictor. 1) How should we select negative samples? 2) What should be the size of negative samples as compared to the positive samples? 3) What type of margin violation penalty should be used to train the predictor? We compare two available methods for negative sampling. Moreover, we propose a new method of assigning weights to each training example in weighted SVM depending on the distance of the negative examples from the positive examples. We have also developed a web server for our HPI predictor called HoPItor (Host Pathogen Interaction predicTOR) that can predict interactions between human and viral proteins. This webserver can be accessed at the URL: http://faculty.pieas.edu.pk/fayyaz/software.html#HoPItor.
pyLEMMINGS: Large Margin Multiple Instance Classification and Ranking for Bioinformatics Applications
Nov 14, 2017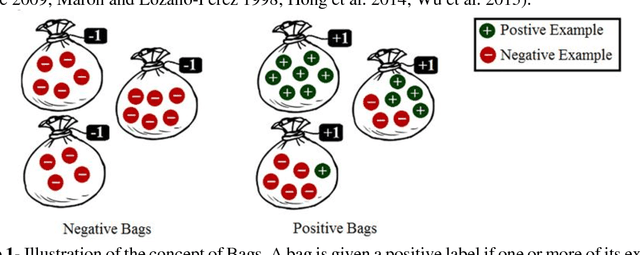

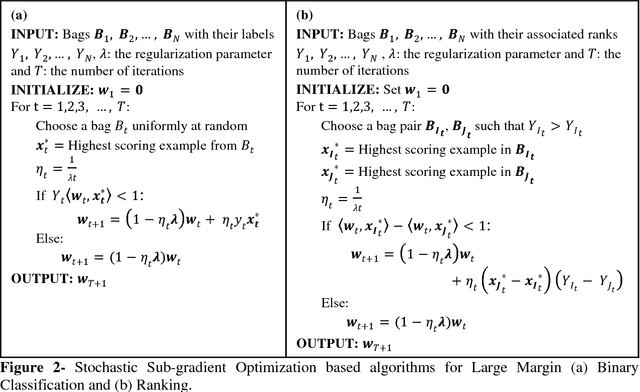
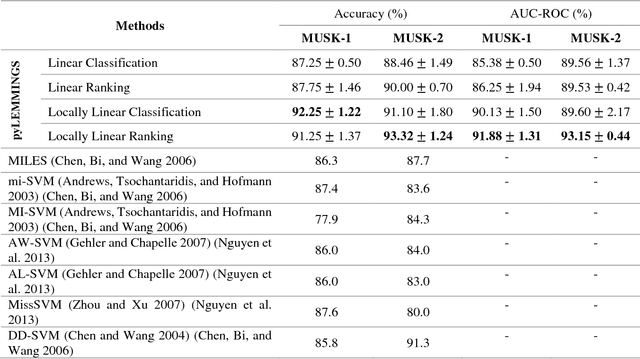
Abstract:Motivation: A major challenge in the development of machine learning based methods in computational biology is that data may not be accurately labeled due to the time and resources required for experimentally annotating properties of proteins and DNA sequences. Standard supervised learning algorithms assume accurate instance-level labeling of training data. Multiple instance learning is a paradigm for handling such labeling ambiguities. However, the widely used large-margin classification methods for multiple instance learning are heuristic in nature with high computational requirements. In this paper, we present stochastic sub-gradient optimization large margin algorithms for multiple instance classification and ranking, and provide them in a software suite called pyLEMMINGS. Results: We have tested pyLEMMINGS on a number of bioinformatics problems as well as benchmark datasets. pyLEMMINGS has successfully been able to identify functionally important segments of proteins: binding sites in Calmodulin binding proteins, prion forming regions, and amyloid cores. pyLEMMINGS achieves state-of-the-art performance in all these tasks, demonstrating the value of multiple instance learning. Furthermore, our method has shown more than 100-fold improvement in terms of running time as compared to heuristic solutions with improved accuracy over benchmark datasets. Availability and Implementation: pyLEMMINGS python package is available for download at: http://faculty.pieas.edu.pk/fayyaz/software.html#pylemmings.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge