Adiba Yaseen
Department of Computer and Information Sciences
Insights into performance evaluation of com-pound-protein interaction prediction methods
Jan 28, 2022
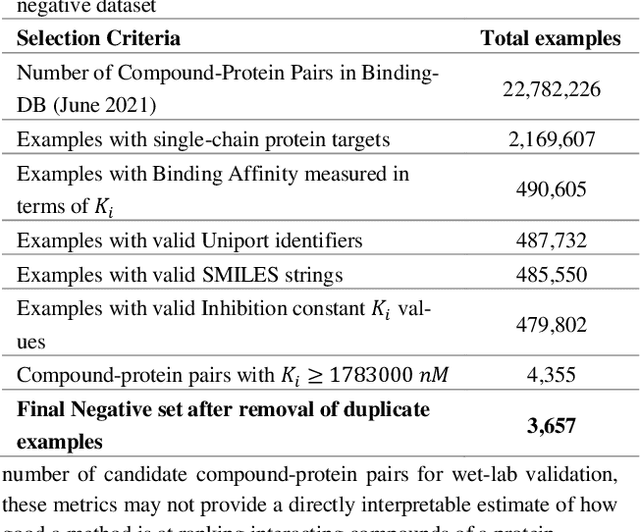
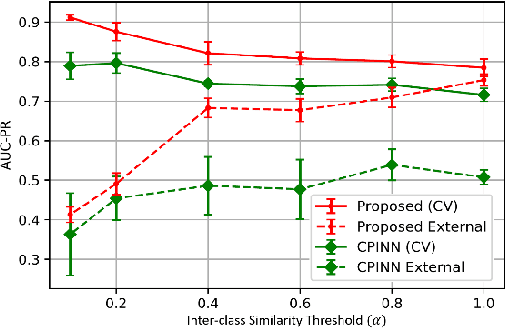
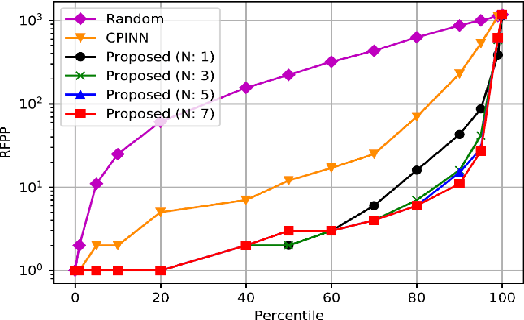
Abstract:Motivation: Machine learning based prediction of compound-protein interactions (CPIs) is important for drug design, screening and repurposing studies and can improve the efficiency and cost-effectiveness of wet lab assays. Despite the publication of many research papers reporting CPI predictors in the recent years, we have observed a number of fundamental issues in experiment design that lead to over optimistic estimates of model performance. Results: In this paper, we analyze the impact of several important factors affecting generalization perfor-mance of CPI predictors that are overlooked in existing work: 1. Similarity between training and test examples in cross-validation 2. The strategy for generating negative examples, in the absence of experimentally verified negative examples. 3. Choice of evaluation protocols and performance metrics and their alignment with real-world use of CPI predictors in screening large compound libraries. Using both an existing state-of-the-art method (CPI-NN) and a proposed kernel based approach, we have found that assessment of predictive performance of CPI predictors requires careful con-trol over similarity between training and test examples. We also show that random pairing for gen-erating synthetic negative examples for training and performance evaluation results in models with better generalization performance in comparison to more sophisticated strategies used in existing studies. Furthermore, we have found that our kernel based approach, despite its simple design, exceeds the prediction performance of CPI-NN. We have used the proposed model for compound screening of several proteins including SARS-CoV-2 Spike and Human ACE2 proteins and found strong evidence in support of its top hits. Availability: Code and raw experimental results available at https://github.com/adibayaseen/HKRCPI Contact: Fayyaz.minhas@warwick.ac.uk
ISLAND: In-Silico Prediction of Proteins Binding Affinity Using Sequence Descriptors
Mar 22, 2018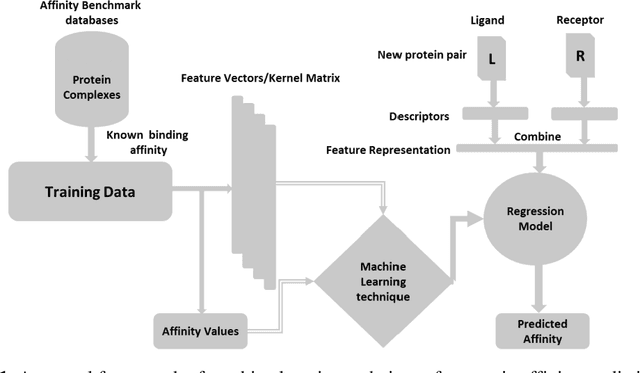
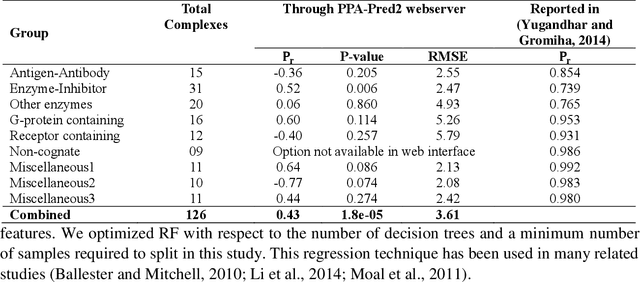
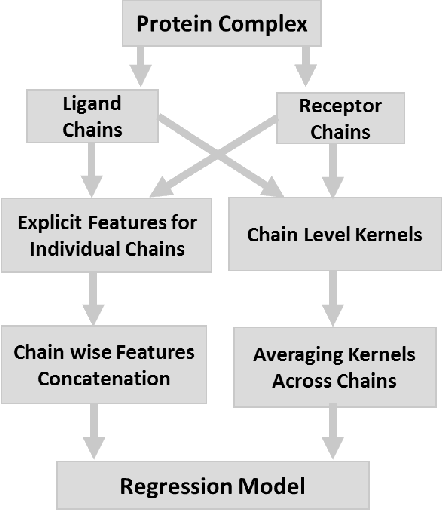
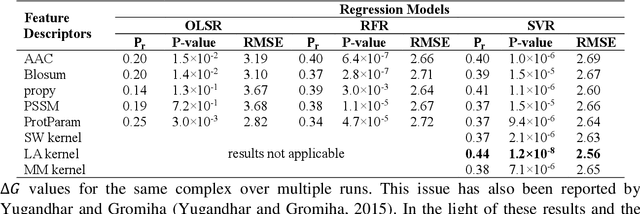
Abstract:Determination of binding affinity of proteins in the formation of protein complexes requires sophisticated, expensive and time-consuming experimentation which can be replaced with computational methods. Most computational prediction techniques require protein structures which limit their applicability to protein complexes with known structures. In this work, we explore sequence based protein binding affinity prediction using machine learning. Our paper highlights the fact that the generalization performance of even the state of the art sequence-only predictor of binding affinity is far from satisfactory and that the development of effective and practical methods in this domain is still an open problem. We also propose a novel sequence-only predictor of binding affinity called ISLAND which gives better accuracy than existing methods over the same validation set as well as on external independent test dataset. A cloud-based webserver implementation of ISLAND and its Python code are available at the URL: http://faculty.pieas.edu.pk/fayyaz/software.html#island.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge