Sven Plein
Unsupervised ensemble-based phenotyping helps enhance the discoverability of genes related to heart morphology
Jan 07, 2023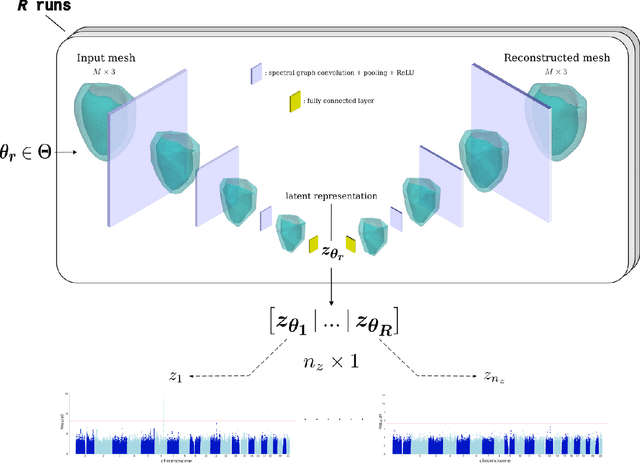
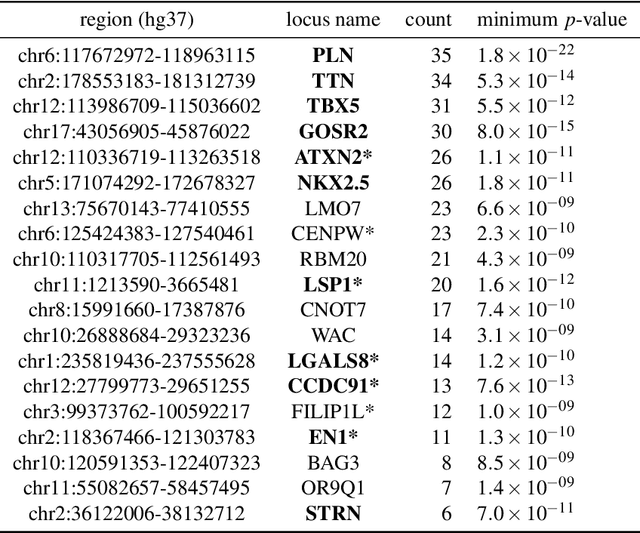
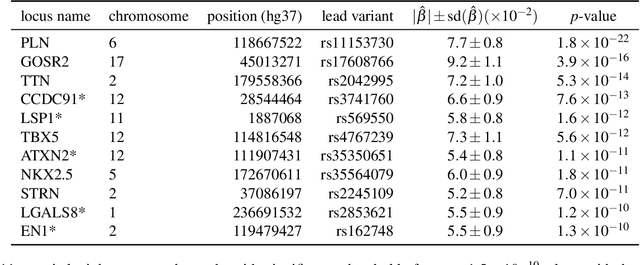
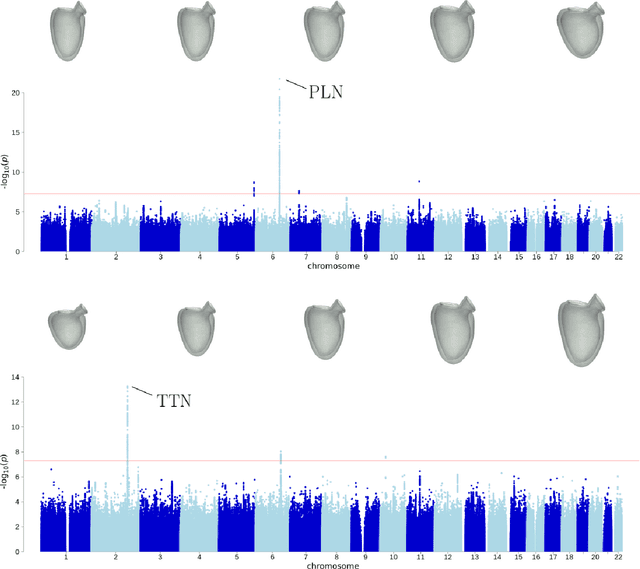
Abstract:Recent genome-wide association studies (GWAS) have been successful in identifying associations between genetic variants and simple cardiac parameters derived from cardiac magnetic resonance (CMR) images. However, the emergence of big databases including genetic data linked to CMR, facilitates investigation of more nuanced patterns of shape variability. Here, we propose a new framework for gene discovery entitled Unsupervised Phenotype Ensembles (UPE). UPE builds a redundant yet highly expressive representation by pooling a set of phenotypes learned in an unsupervised manner, using deep learning models trained with different hyperparameters. These phenotypes are then analyzed via (GWAS), retaining only highly confident and stable associations across the ensemble. We apply our approach to the UK Biobank database to extract left-ventricular (LV) geometric features from image-derived three-dimensional meshes. We demonstrate that our approach greatly improves the discoverability of genes influencing LV shape, identifying 11 loci with study-wide significance and 8 with suggestive significance. We argue that our approach would enable more extensive discovery of gene associations with image-derived phenotypes for other organs or image modalities.
Automated Inline Analysis of Myocardial Perfusion MRI with Deep Learning
Nov 02, 2019



Abstract:Recent development of quantitative myocardial blood flow (MBF) mapping allows direct evaluation of absolute myocardial perfusion, by computing pixel-wise flow maps. Clinical studies suggest quantitative evaluation would be more desirable for objectivity and efficiency. Objective assessment can be further facilitated by segmenting the myocardium and automatically generating reports following the AHA model. This will free user interaction for analysis and lead to a 'one-click' solution to improve workflow. This paper proposes a deep neural network based computational workflow for inline myocardial perfusion analysis. Adenosine stress and rest perfusion scans were acquired from three hospitals. Training set included N=1,825 perfusion series from 1,034 patients. Independent test set included 200 scans from 105 patients. Data were consecutively acquired at each site. A convolution neural net (CNN) model was trained to provide segmentation for LV cavity, myocardium and right ventricular by processing incoming 2D+T perfusion Gd series. Model outputs were compared to manual ground-truth for accuracy of segmentation and flow measures derived on global and per-sector basis. The trained models were integrated onto MR scanners for effective inference. Segmentation accuracy and myocardial flow measures were compared between CNN models and manual ground-truth. The mean Dice ratio of CNN derived myocardium was 0.93 +/- 0.04. Both global flow and per-sector values showed no significant difference, compared to manual results. The AHA 16 segment model was automatically generated and reported on the MR scanner. As a result, the fully automated analysis of perfusion flow mapping was achieved. This solution was integrated on the MR scanner, enabling 'one-click' analysis and reporting of myocardial blood flow.
Automated Detection of Left Ventricle in Arterial Input Function Images for Inline Perfusion Mapping using Deep Learning: A study of 15,000 Patients
Oct 16, 2019


Abstract:Quantification of myocardial perfusion has the potential to improve detection of regional and global flow reduction. Significant effort has been made to automate the workflow, where one essential step is the arterial input function (AIF) extraction. Since failure here invalidates quantification, high accuracy is required. For this purpose, this study presents a robust AIF detection method using the convolutional neural net (CNN) model. CNN models were trained by assembling 25,027 scans (N=12,984 patients) from three hospitals, seven scanners. A test set of 5,721 scans (N=2,805 patients) evaluated model performance. The 2D+T AIF time series was inputted into CNN. Two variations were investigated: a) Two Classes (2CS) for background and foreground (LV mask); b) Three Classes (3CS) for background, foreground LV and RV. Final model was deployed on MR scanners via the Gadgetron InlineAI. Model loading time on MR scanner was ~340ms and applying it took ~180ms. The 3CS model successfully detect LV for 99.98% of all test cases (1 failed out of 5,721 cases). The mean Dice ratio for 3CS was 0.87+/-0.08 with 92.0% of all test cases having Dice ratio >0.75, while the 2CS model gave lower Dice of 0.82+/-0.22 (P<1e-5). Extracted AIF signals using CNN were further compared to manual ground-truth for foot-time, peak-time, first-pass duration, peak value and area-under-curve. No significant differences were found for all features (P>0.2). This study proposed, validated, and deployed a robust CNN solution to detect the LV for the extraction of the AIF signal used in fully automated perfusion flow mapping. A very large data cohort was assembled and resulting models were deployed to MR scanners for fully inline AI in clinical hospitals.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge