Sila Kurugol
An Optimized Binning and Probabilistic Slice Sharing Algorithm for Motion Correction in Abdominal DW-MRI
Sep 01, 2024Abstract:Abdominal diffusion-weighted magnetic resonance imaging (DW-MRI) is a powerful, non-invasive technique for characterizing lesions and facilitating early diagnosis. However, respiratory motion during a scan can degrade image quality. Binning image slices into respiratory phases may reduce motion artifacts, but when the standard binning algorithm is applied to DW-MRI, reconstructed volumes are often incomplete because they lack slices along the superior-inferior axis. Missing slices create black stripes within images, and prolonged scan times are required to generate complete volumes. In this study, we propose a new binning algorithm to minimize missing slices. We acquired free-breathing and shallow-breathing abdominal DW-MRI scans on seven volunteers and used our algorithm to correct for motion in free-breathing scans. First, we drew the optimal rigid bin partitions in the respiratory signal using a dynamic programming approach, assigning each slice to one bin. We then designed a probabilistic approach for selecting some slices to belong in two bins. Our proposed binning algorithm resulted in significantly fewer missing slices than standard binning (p<1.0e-16), yielding an average reduction of 82.98+/-6.07%. Our algorithm also improved lesion conspicuity and reduced motion artifacts in DW-MR images and Apparent Diffusion Coefficient (ADC) maps. ADC maps created from free-breathing images corrected for motion with our algorithm showed lower intra-subject variability compared to uncorrected free-breathing and shallow-breathing maps (p<0.001). Additionally, shallow-breathing ADC maps showed more consistency with corrected free-breathing maps rather than uncorrected free-breathing maps (p<0.01). Our proposed binning algorithm's efficacy in reducing missing slices increases anatomical accuracy and allows for shorter acquisition times compared to standard binning.
IVIM-Morph: Motion-compensated quantitative Intra-voxel Incoherent Motion (IVIM) analysis for functional fetal lung maturity assessment from diffusion-weighted MRI data
Jan 17, 2024
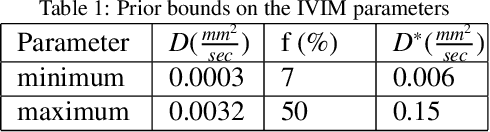

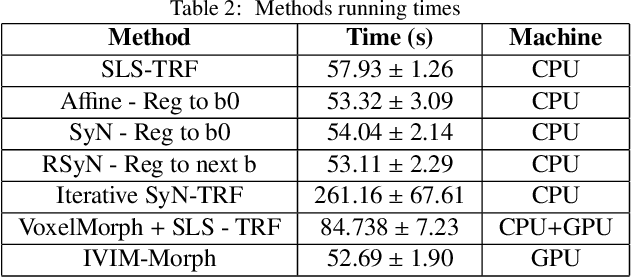
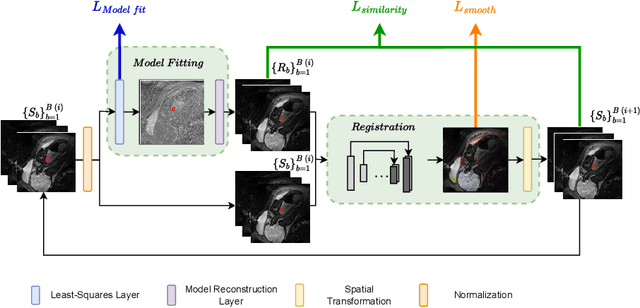
Abstract:Quantitative analysis of pseudo-diffusion in diffusion-weighted magnetic resonance imaging (DWI) data shows potential for assessing fetal lung maturation and generating valuable imaging biomarkers. Yet, the clinical utility of DWI data is hindered by unavoidable fetal motion during acquisition. We present IVIM-morph, a self-supervised deep neural network model for motion-corrected quantitative analysis of DWI data using the Intra-voxel Incoherent Motion (IVIM) model. IVIM-morph combines two sub-networks, a registration sub-network, and an IVIM model fitting sub-network, enabling simultaneous estimation of IVIM model parameters and motion. To promote physically plausible image registration, we introduce a biophysically informed loss function that effectively balances registration and model-fitting quality. We validated the efficacy of IVIM-morph by establishing a correlation between the predicted IVIM model parameters of the lung and gestational age (GA) using fetal DWI data of 39 subjects. IVIM-morph exhibited a notably improved correlation with gestational age (GA) when performing in-vivo quantitative analysis of fetal lung DWI data during the canalicular phase. IVIM-morph shows potential in developing valuable biomarkers for non-invasive assessment of fetal lung maturity with DWI data. Moreover, its adaptability opens the door to potential applications in other clinical contexts where motion compensation is essential for quantitative DWI analysis. The IVIM-morph code is readily available at: https://github.com/TechnionComputationalMRILab/qDWI-Morph.
Masked Conditional Diffusion Models for Image Analysis with Application to Radiographic Diagnosis of Infant Abuse
Nov 22, 2023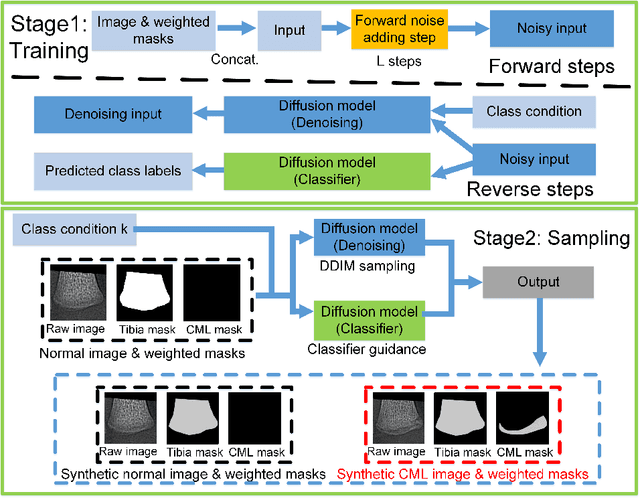
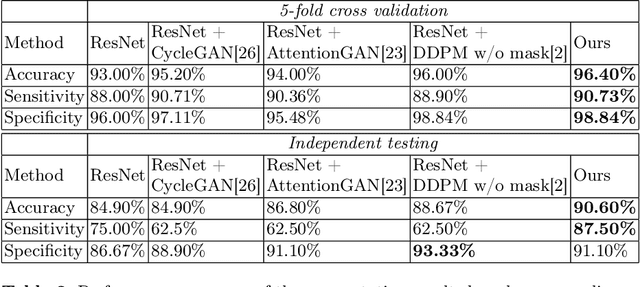
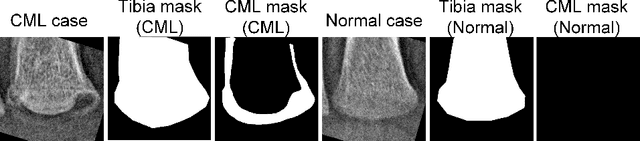

Abstract:The classic metaphyseal lesion (CML) is a distinct injury that is highly specific for infant abuse. It commonly occurs in the distal tibia. To aid radiologists detect these subtle fractures, we need to develop a model that can flag abnormal distal tibial radiographs (i.e. those with CMLs). Unfortunately, the development of such a model requires a large and diverse training database, which is often not available. To address this limitation, we propose a novel generative model for data augmentation. Unlike previous models that fail to generate data that span the diverse radiographic appearance of the distal tibial CML, our proposed masked conditional diffusion model (MaC-DM) not only generates realistic-appearing and wide-ranging synthetic images of the distal tibial radiographs with and without CMLs, it also generates their associated segmentation labels. To achieve these tasks, MaC-DM combines the weighted segmentation masks of the tibias and the CML fracture sites as additional conditions for classifier guidance. The augmented images from our model improved the performances of ResNet-34 in classifying normal radiographs and those with CMLs. Further, the augmented images and their associated segmentation masks enhanced the performance of the U-Net in labeling areas of the CMLs on distal tibial radiographs.
qDWI-Morph: Motion-compensated quantitative Diffusion-Weighted MRI analysis for fetal lung maturity assessment
Aug 21, 2022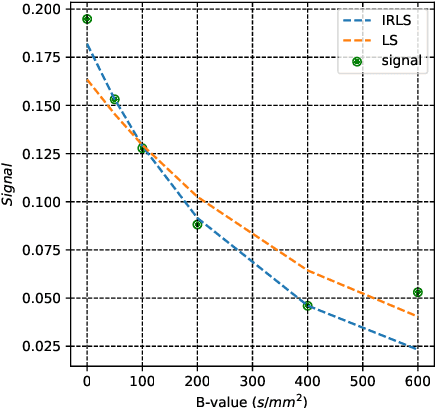

Abstract:Quantitative analysis of fetal lung Diffusion-Weighted MRI (DWI) data shows potential in providing quantitative imaging biomarkers that indirectly reflect fetal lung maturation. However, fetal motion during the acquisition hampered quantitative analysis of the acquired DWI data and, consequently, reliable clinical utilization. We introduce qDWI-morph, an unsupervised deep-neural-network architecture for motion compensated quantitative DWI (qDWI) analysis. Our approach couples a registration sub-network with a quantitative DWI model fitting sub-network. We simultaneously estimate the qDWI parameters and the motion model by minimizing a bio-physically-informed loss function integrating a registration loss and a model fitting quality loss. We demonstrated the added-value of qDWI-morph over: 1) a baseline qDWI analysis without motion compensation and 2) a baseline deep-learning model incorporating registration loss solely. The qDWI-morph achieved a substantially improved correlation with the gestational age through in-vivo qDWI analysis of fetal lung DWI data (R-squared=0.32 vs. 0.13, 0.28). Our qDWI-morph has the potential to enable motion-compensated quantitative analysis of DWI data and to provide clinically feasible bio-markers for non-invasive fetal lung maturity assessment. Our code is available at: https://github.com/TechnionComputationalMRILab/qDWI-Morph.
SUPER-IVIM-DC: Intra-voxel incoherent motion based Fetal lung maturity assessment from limited DWI data using supervised learning coupled with data-consistency
Jun 19, 2022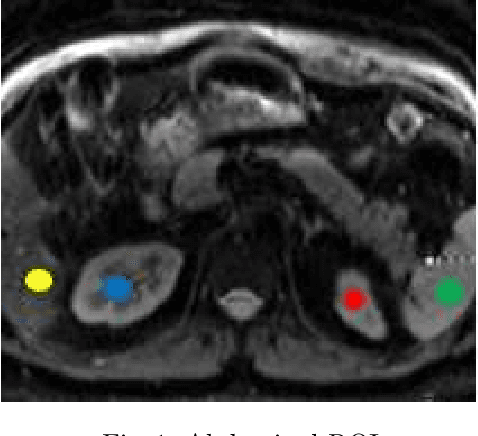
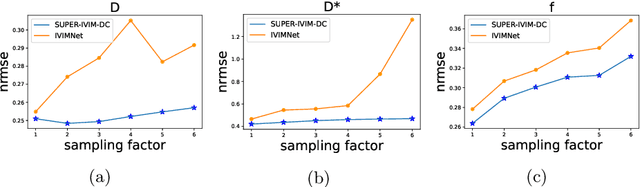
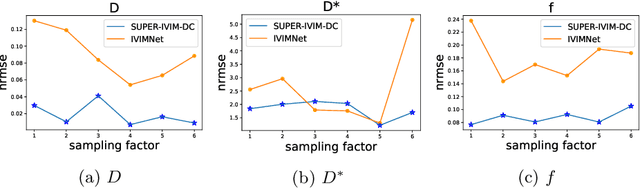
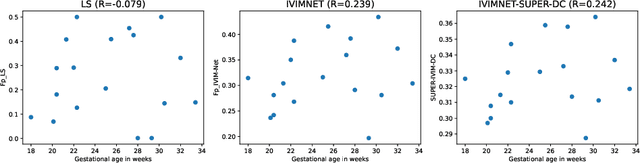
Abstract:Intra-voxel incoherent motion (IVIM) analysis of fetal lungs Diffusion-Weighted MRI (DWI) data shows potential in providing quantitative imaging bio-markers that reflect, indirectly, diffusion and pseudo-diffusion for non-invasive fetal lung maturation assessment. However, long acquisition times, due to the large number of different "b-value" images required for IVIM analysis, precluded clinical feasibility. We introduce SUPER-IVIM-DC a deep-neural-networks (DNN) approach which couples supervised loss with a data-consistency term to enable IVIM analysis of DWI data acquired with a limited number of b-values. We demonstrated the added-value of SUPER-IVIM-DC over both classical and recent DNN approaches for IVIM analysis through numerical simulations, healthy volunteer study, and IVIM analysis of fetal lung maturation from fetal DWI data. Our numerical simulations and healthy volunteer study show that SUPER-IVIM-DC estimates of the IVIM model parameters from limited DWI data had lower normalized root mean-squared error compared to previous DNN-based approaches. Further, SUPER-IVIM-DC estimates of the pseudo-diffusion fraction parameter from limited DWI data of fetal lungs correlate better with gestational age compared to both to classical and DNN-based approaches (0.242 vs. -0.079 and 0.239). SUPER-IVIM-DC has the potential to reduce the long acquisition times associated with IVIM analysis of DWI data and to provide clinically feasible bio-markers for non-invasive fetal lung maturity assessment.
CORPS: Cost-free Rigorous Pseudo-labeling based on Similarity-ranking for Brain MRI Segmentation
May 19, 2022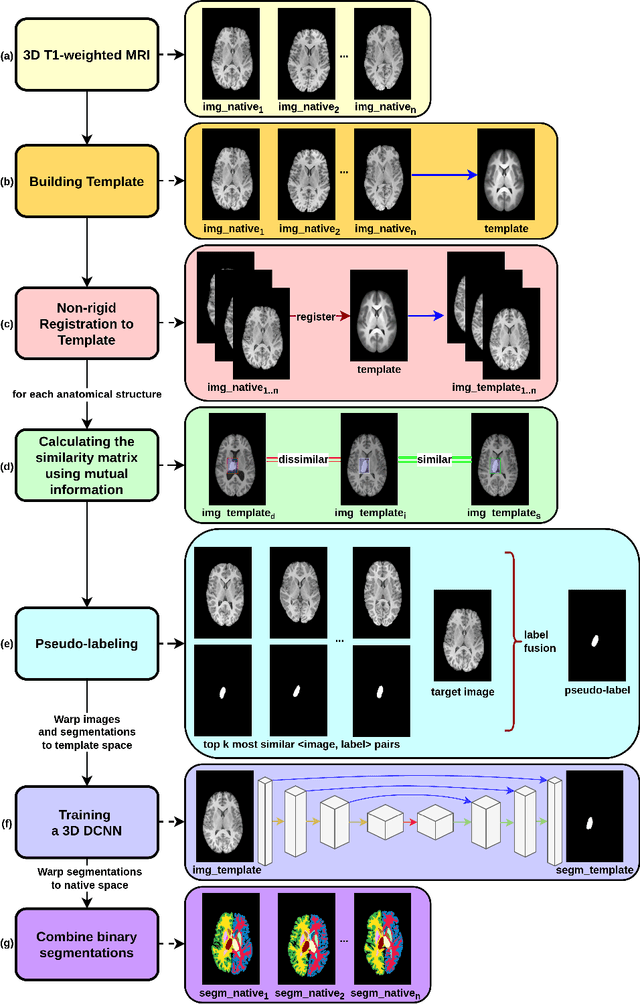
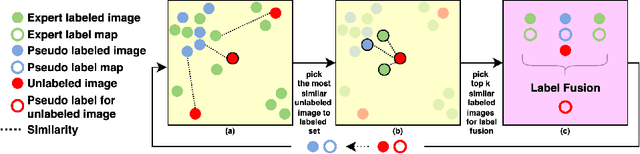
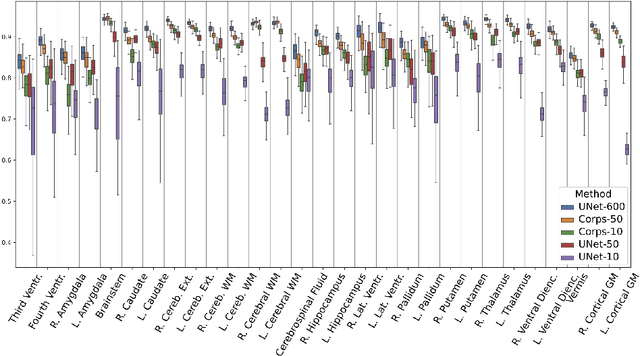
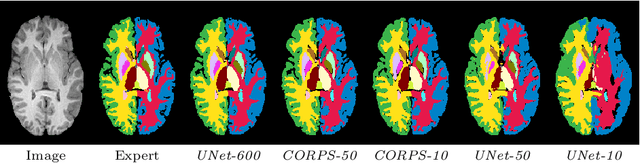
Abstract:Segmentation of brain magnetic resonance images (MRI) is crucial for the analysis of the human brain and diagnosis of various brain disorders. The drawbacks of time-consuming and error-prone manual delineation procedures are aimed to be alleviated by atlas-based and supervised machine learning methods where the former methods are computationally intense and the latter methods lack a sufficiently large number of labeled data. With this motivation, we propose CORPS, a semi-supervised segmentation framework built upon a novel atlas-based pseudo-labeling method and a 3D deep convolutional neural network (DCNN) for 3D brain MRI segmentation. In this work, we propose to generate expert-level pseudo-labels for unlabeled set of images in an order based on a local intensity-based similarity score to existing labeled set of images and using a novel atlas-based label fusion method. Then, we propose to train a 3D DCNN on the combination of expert and pseudo labeled images for binary segmentation of each anatomical structure. The binary segmentation approach is proposed to avoid the poor performance of multi-class segmentation methods on limited and imbalanced data. This also allows to employ a lightweight and efficient 3D DCNN in terms of the number of filters and reserve memory resources for training the binary networks on full-scale and full-resolution 3D MRI volumes instead of 2D/3D patches or 2D slices. Thus, the proposed framework can encapsulate the spatial contiguity in each dimension and enhance context-awareness. The experimental results demonstrate the superiority of the proposed framework over the baseline method both qualitatively and quantitatively without additional labeling cost for manual labeling.
Learning the Regularization in DCE-MR Image Reconstruction for Functional Imaging of Kidneys
Sep 15, 2021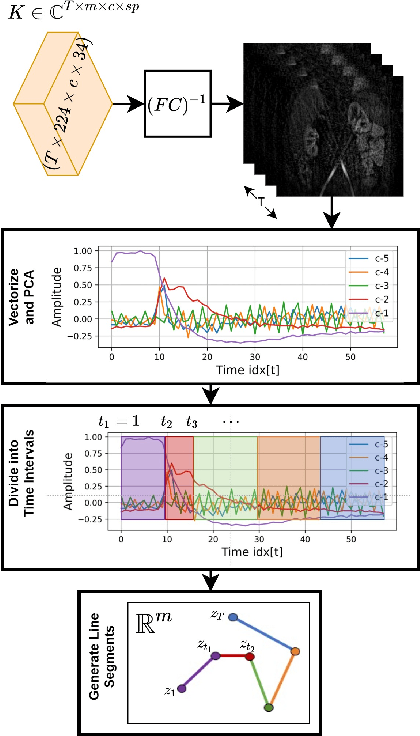
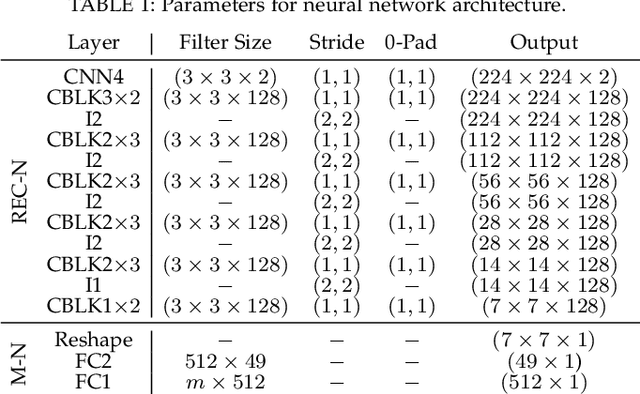
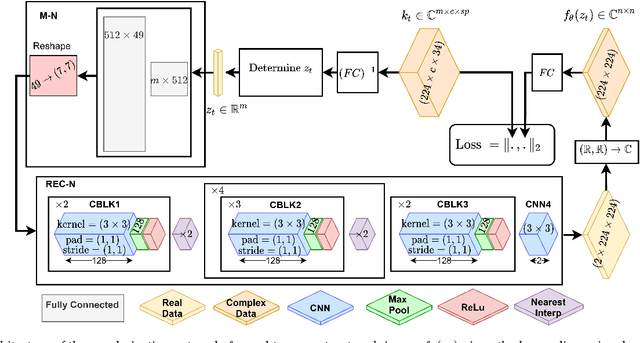
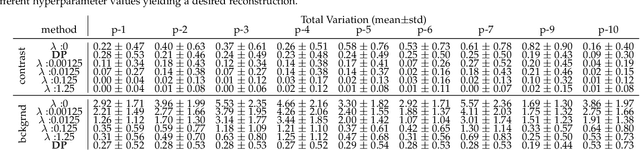
Abstract:Kidney DCE-MRI aims at both qualitative assessment of kidney anatomy and quantitative assessment of kidney function by estimating the tracer kinetic (TK) model parameters. Accurate estimation of TK model parameters requires an accurate measurement of the arterial input function (AIF) with high temporal resolution. Accelerated imaging is used to achieve high temporal resolution, which yields under-sampling artifacts in the reconstructed images. Compressed sensing (CS) methods offer a variety of reconstruction options. Most commonly, sparsity of temporal differences is encouraged for regularization to reduce artifacts. Increasing regularization in CS methods removes the ambient artifacts but also over-smooths the signal temporally which reduces the parameter estimation accuracy. In this work, we propose a single image trained deep neural network to reduce MRI under-sampling artifacts without reducing the accuracy of functional imaging markers. Instead of regularizing with a penalty term in optimization, we promote regularization by generating images from a lower dimensional representation. In this manuscript we motivate and explain the lower dimensional input design. We compare our approach to CS reconstructions with multiple regularization weights. Proposed approach results in kidney biomarkers that are highly correlated with the ground truth markers estimated using the CS reconstruction which was optimized for functional analysis. At the same time, the proposed approach reduces the artifacts in the reconstructed images.
Weakly Supervised Segmentation by A Deep Geodesic Prior
Aug 18, 2019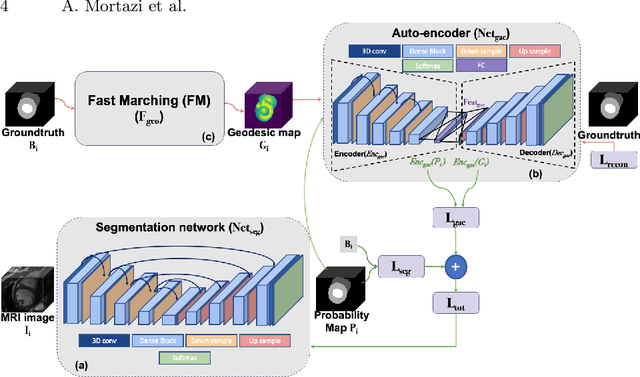
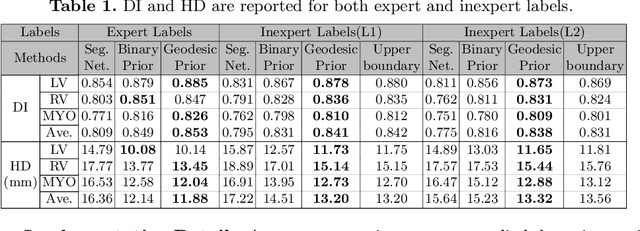
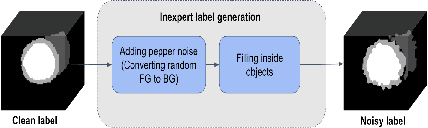
Abstract:The performance of the state-of-the-art image segmentation methods heavily relies on the high-quality annotations, which are not easily affordable, particularly for medical data. To alleviate this limitation, in this study, we propose a weakly supervised image segmentation method based on a deep geodesic prior. We hypothesize that integration of this prior information can reduce the adverse effects of weak labels in segmentation accuracy. Our proposed algorithm is based on a prior information, extracted from an auto-encoder, trained to map objects geodesic maps to their corresponding binary maps. The obtained information is then used as an extra term in the loss function of the segmentor. In order to show efficacy of the proposed strategy, we have experimented segmentation of cardiac substructures with clean and two levels of noisy labels (L1, L2). Our experiments showed that the proposed algorithm boosted the performance of baseline deep learning-based segmentation for both clean and noisy labels by 4.4%, 4.6%(L1), and 6.3%(L2) in dice score, respectively. We also showed that the proposed method was more robust in the presence of high-level noise due to the existence of shape priors.
Automatic Renal Segmentation in DCE-MRI using Convolutional Neural Networks
Dec 19, 2017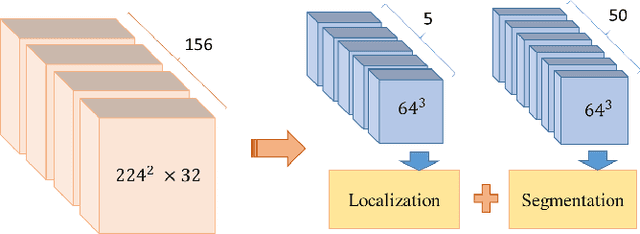
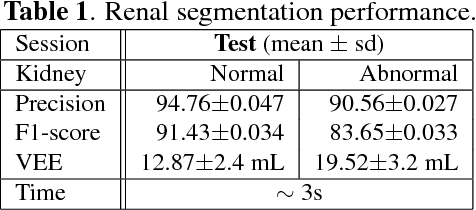
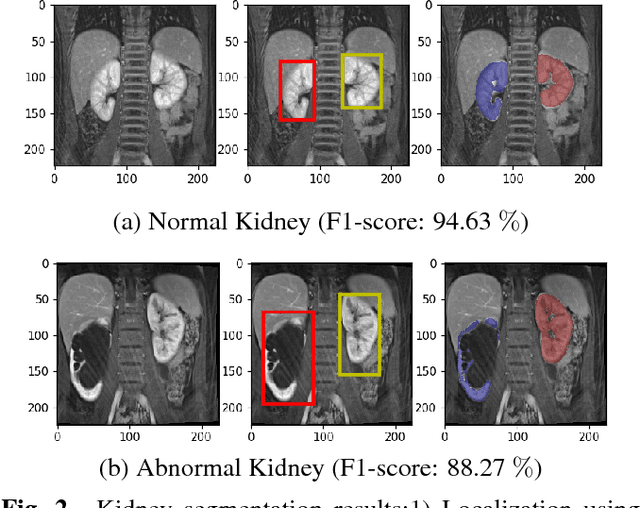
Abstract:Kidney function evaluation using dynamic contrast-enhanced MRI (DCE-MRI) images could help in diagnosis and treatment of kidney diseases of children. Automatic segmentation of renal parenchyma is an important step in this process. In this paper, we propose a time and memory efficient fully automated segmentation method which achieves high segmentation accuracy with running time in the order of seconds in both normal kidneys and kidneys with hydronephrosis. The proposed method is based on a cascaded application of two 3D convolutional neural networks that employs spatial and temporal information at the same time in order to learn the tasks of localization and segmentation of kidneys, respectively. Segmentation performance is evaluated on both normal and abnormal kidneys with varying levels of hydronephrosis. We achieved a mean dice coefficient of 91.4 and 83.6 for normal and abnormal kidneys of pediatric patients, respectively.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge