Shigao Chen
Contrast-Free Ultrasound Microvascular Imaging via Radiality and Similarity Weighting
Sep 08, 2025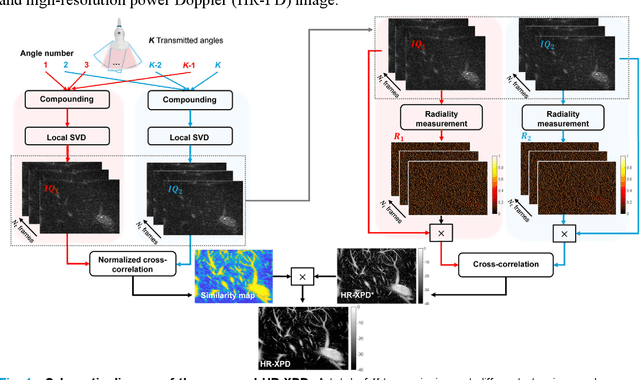
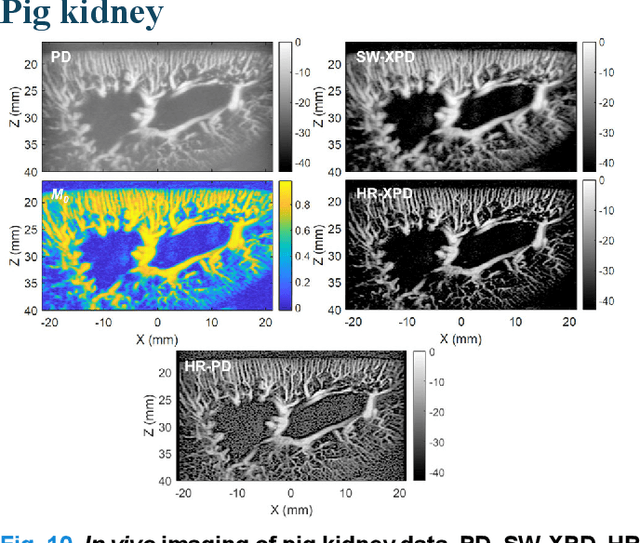
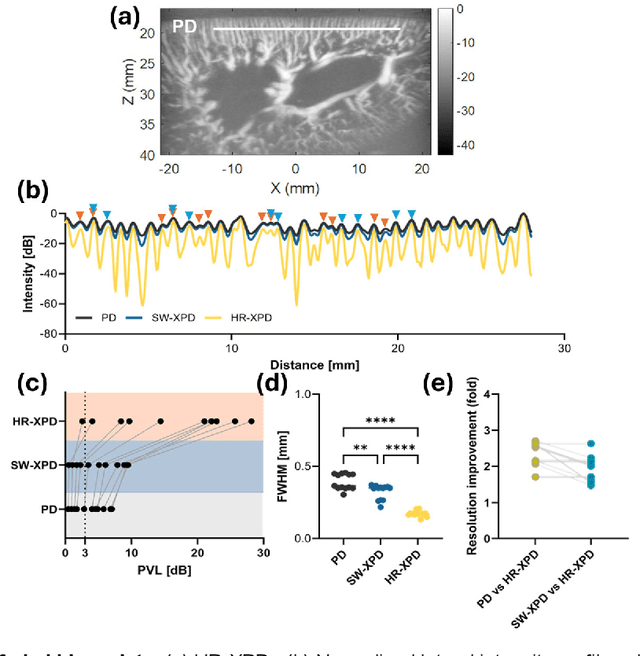
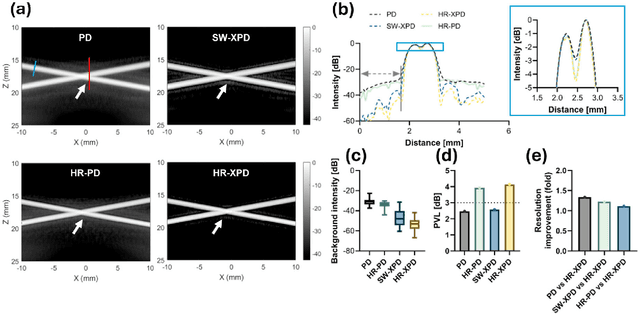
Abstract:Microvascular imaging has advanced significantly with ultrafast data acquisition and improved clutter filtering, enhancing the sensitivity of power Doppler imaging to small vessels. However, the image quality remains limited by spatial resolution and elevated background noise, both of which impede visualization and accurate quantification. To address these limitations, this study proposes a high-resolution cross-correlation Power Doppler (HR-XPD) method that integrates spatial radiality weighting with Doppler signal coherence analysis, thereby enhancing spatial resolution while suppressing artifacts and background noise. Quantitative evaluations in simulation and in vivo experiments on healthy human liver, transplanted human kidney, and pig kidney demonstrated that HR-XPD significantly improves microvascular resolvability and contrast compared to conventional PD. In vivo results showed up to a 2 to 3-fold enhancement in spatial resolution and an increase in contrast by up to 20 dB. High-resolution vascular details were clearly depicted within a short acquisition time of only 0.3 s-1.2 s without the use of contrast agents. These findings indicate that HR-XPD provides an effective, contrast-free, and high-resolution microvascular imaging approach with broad applicability in both preclinical and clinical research.
Optimizing In Vivo Data Acquisition for Robust Clinical Microvascular Imaging Using Ultrasound Localization Microscopy
Dec 24, 2024



Abstract:Ultrasound localization microscopy (ULM) enables microvascular imaging at spatial resolutions beyond the acoustic diffraction limit, offering significant clinical potentials. However, ULM performance relies heavily on microbubble (MB) signal sparsity, the number of detected MBs, and signal-to-noise ratio (SNR), all of which vary in clinical scenarios involving bolus MB injections. These sources of variations underscore the need to optimize MB dosage, data acquisition timing, and imaging settings in order to standardize and optimize ULM of microvasculature. This pilot study investigated temporal changes in MB signals during bolus injections in both pig and human models to optimize data acquisition for clinical ULM. Quantitative indices were developed to evaluate MB signal quality, guiding selection of acquisition timing that balances the MB localization quality and adequate MB counts. The effects of transmitted voltage and dosage were also explored. In the pig model, a relatively short window (approximately 10 seconds) for optimal acquisition was identified during the rapid wash-out phase, highlighting the need for real-time MB signal monitoring during data acquisition. The slower wash-out phase in humans allowed for a more flexible imaging window of 1-2 minutes, while trade-offs were observed between localization quality and MB density (or acquisition length) at different wash-out phase timings. Guided by these findings, robust ULM imaging was achieved in both pig and human kidneys using a short period of data acquisition, demonstrating its feasibility in clinical practice. This study provides insights into optimizing data acquisition for consistent and reproducible ULM, paving the way for its standardization and broader clinical applications.
Joint localization and classification of breast tumors on ultrasound images using a novel auxiliary attention-based framework
Oct 11, 2022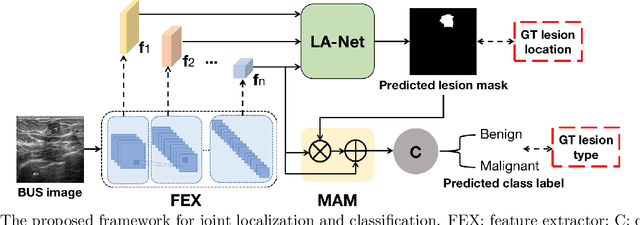
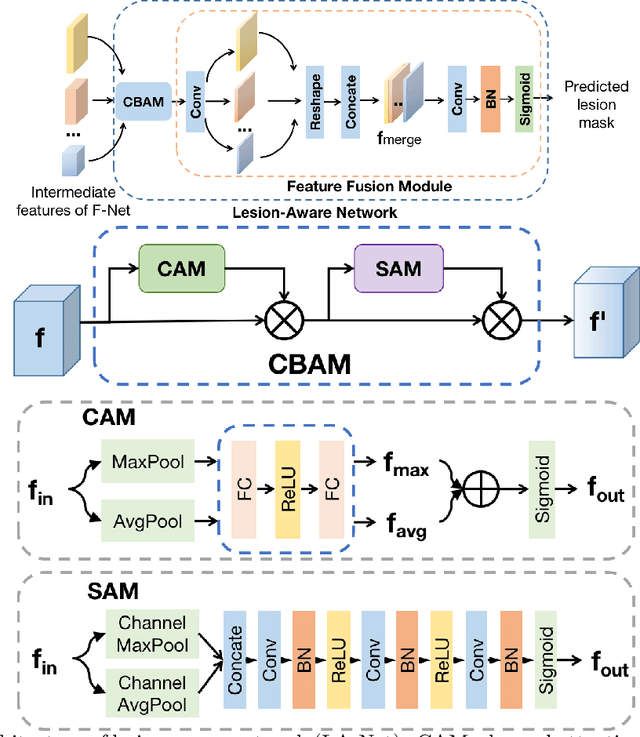
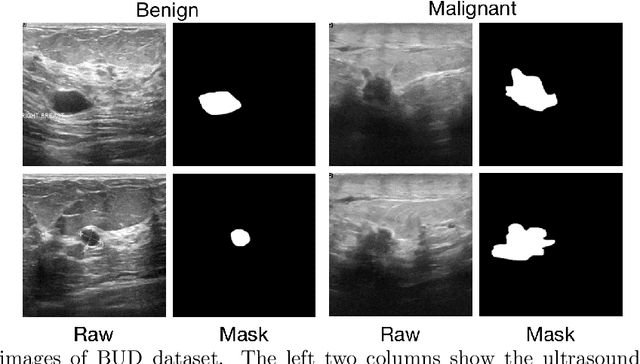
Abstract:Automatic breast lesion detection and classification is an important task in computer-aided diagnosis, in which breast ultrasound (BUS) imaging is a common and frequently used screening tool. Recently, a number of deep learning-based methods have been proposed for joint localization and classification of breast lesions using BUS images. In these methods, features extracted by a shared network trunk are appended by two independent network branches to achieve classification and localization. Improper information sharing might cause conflicts in feature optimization in the two branches and leads to performance degradation. Also, these methods generally require large amounts of pixel-level annotated data for model training. To overcome these limitations, we proposed a novel joint localization and classification model based on the attention mechanism and disentangled semi-supervised learning strategy. The model used in this study is composed of a classification network and an auxiliary lesion-aware network. By use of the attention mechanism, the auxiliary lesion-aware network can optimize multi-scale intermediate feature maps and extract rich semantic information to improve classification and localization performance. The disentangled semi-supervised learning strategy only requires incomplete training datasets for model training. The proposed modularized framework allows flexible network replacement to be generalized for various applications. Experimental results on two different breast ultrasound image datasets demonstrate the effectiveness of the proposed method. The impacts of various network factors on model performance are also investigated to gain deep insights into the designed framework.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge