Sebastian Bauer
Real-time Non-line-of-Sight imaging of dynamic scenes
Oct 24, 2020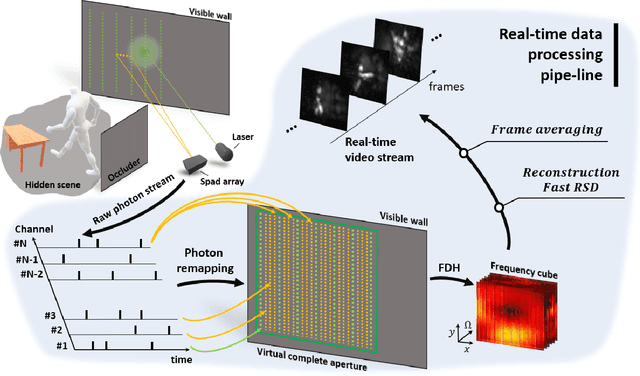
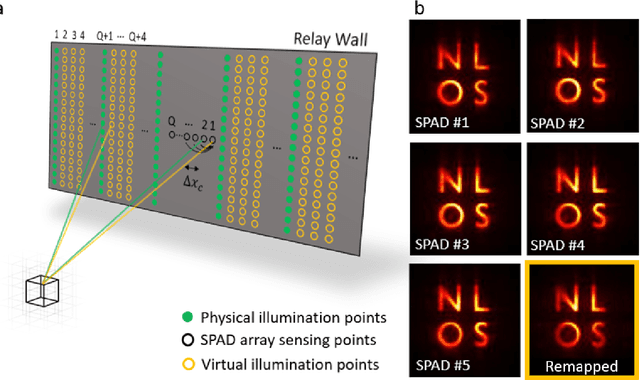
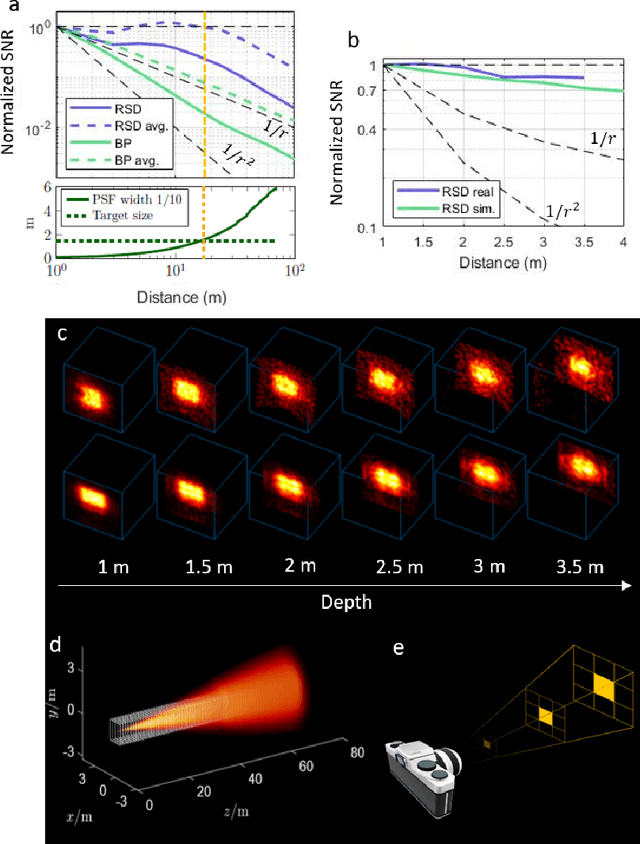
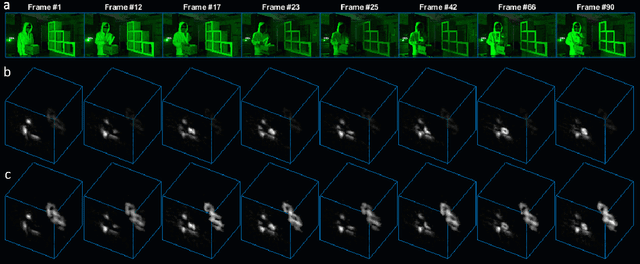
Abstract:Non-Line-of-Sight (NLOS) imaging aims at recovering the 3D geometry of objects that are hidden from the direct line of sight. In the past, this method has suffered from the weak available multibounce signal limiting scene size, capture speed, and reconstruction quality. While algorithms capable of reconstructing scenes at several frames per second have been demonstrated, real-time NLOS video has only been demonstrated for retro-reflective objects where the NLOS signal strength is enhanced by 4 orders of magnitude or more. Furthermore, it has also been noted that the signal-to-noise ratio of reconstructions in NLOS methods drops quickly with distance and past reconstructions, therefore, have been limited to small scenes with depths of few meters. Actual models of noise and resolution in the scene have been simplistic, ignoring many of the complexities of the problem. We show that SPAD (Single-Photon Avalanche Diode) array detectors with a total of just 28 pixels combined with a specifically extended Phasor Field reconstruction algorithm can reconstruct live real-time videos of non-retro-reflective NLOS scenes. We provide an analysis of the Signal-to-Noise-Ratio (SNR) of our reconstructions and show that for our method it is possible to reconstruct the scene such that SNR, motion blur, angular resolution, and depth resolution are all independent of scene size suggesting that reconstruction of very large scenes may be possible. In the future, the light efficiency for NLOS imaging systems can be improved further by adding more pixels to the sensor array.
Staging Epileptogenesis with Deep Neural Networks
Jun 17, 2020



Abstract:Epilepsy is a common neurological disorder characterized by recurrent seizures accompanied by excessive synchronous brain activity. The process of structural and functional brain alterations leading to increased seizure susceptibility and eventually spontaneous seizures is called epileptogenesis (EPG) and can span months or even years. Detecting and monitoring the progression of EPG could allow for targeted early interventions that could slow down disease progression or even halt its development. Here, we propose an approach for staging EPG using deep neural networks and identify potential electroencephalography (EEG) biomarkers to distinguish different phases of EPG. Specifically, continuous intracranial EEG recordings were collected from a rodent model where epilepsy is induced by electrical perforant pathway stimulation (PPS). A deep neural network (DNN) is trained to distinguish EEG signals from before stimulation (baseline), shortly after the PPS and long after the PPS but before the first spontaneous seizure (FSS). Experimental results show that our proposed method can classify EEG signals from the three phases with an average area under the curve (AUC) of 0.93, 0.89, and 0.86. To the best of our knowledge, this represents the first successful attempt to stage EPG prior to the FSS using DNNs.
Towards Early Diagnosis of Epilepsy from EEG Data
Jun 17, 2020



Abstract:Epilepsy is one of the most common neurological disorders, affecting about 1% of the population at all ages. Detecting the development of epilepsy, i.e., epileptogenesis (EPG), before any seizures occur could allow for early interventions and potentially more effective treatments. Here, we investigate if modern machine learning (ML) techniques can detect EPG from intra-cranial electroencephalography (EEG) recordings prior to the occurrence of any seizures. For this we use a rodent model of epilepsy where EPG is triggered by electrical stimulation of the brain. We propose a ML framework for EPG identification, which combines a deep convolutional neural network (CNN) with a prediction aggregation method to obtain the final classification decision. Specifically, the neural network is trained to distinguish five second segments of EEG recordings taken from either the pre-stimulation period or the post-stimulation period. Due to the gradual development of epilepsy, there is enormous overlap of the EEG patterns before and after the stimulation. Hence, a prediction aggregation process is introduced, which pools predictions over a longer period. By aggregating predictions over one hour, our approach achieves an area under the curve (AUC) of 0.99 on the EPG detection task. This demonstrates the feasibility of EPG prediction from EEG recordings.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge