Diyuan Lu
Multiple Instance Learning for Brain Tumor Detection from Magnetic Resonance Spectroscopy Data
Dec 16, 2021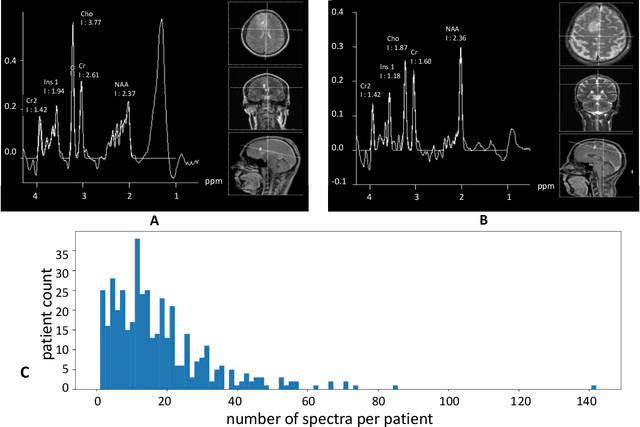
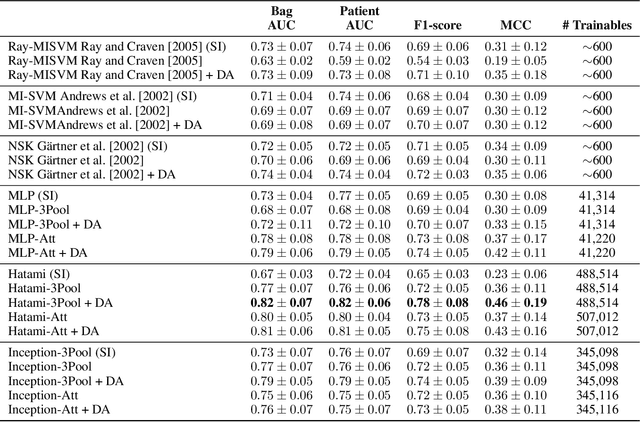
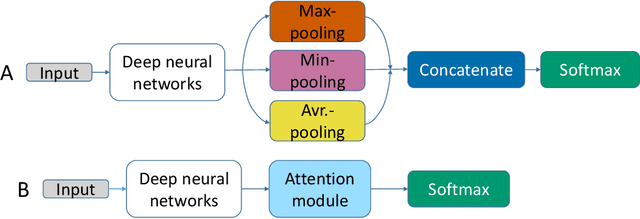
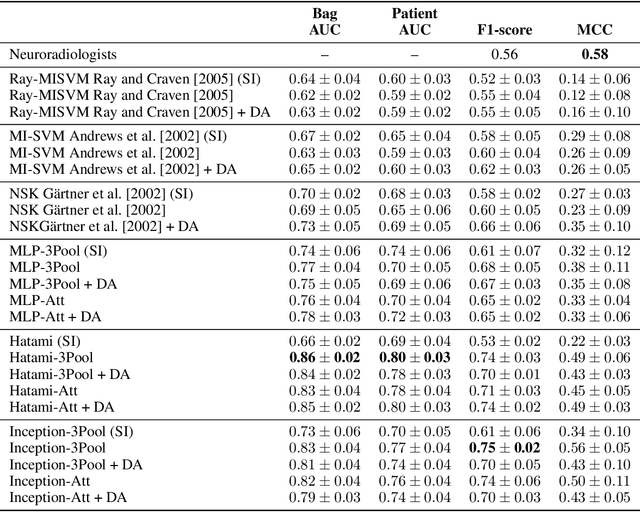
Abstract:We apply deep learning (DL) on Magnetic resonance spectroscopy (MRS) data for the task of brain tumor detection. Medical applications often suffer from data scarcity and corruption by noise. Both of these problems are prominent in our data set. Furthermore, a varying number of spectra are available for the different patients. We address these issues by considering the task as a multiple instance learning (MIL) problem. Specifically, we aggregate multiple spectra from the same patient into a "bag" for classification and apply data augmentation techniques. To achieve the permutation invariance during the process of bagging, we proposed two approaches: (1) to apply min-, max-, and average-pooling on the features of all samples in one bag and (2) to apply an attention mechanism. We tested these two approaches on multiple neural network architectures. We demonstrate that classification performance is significantly improved when training on multiple instances rather than single spectra. We propose a simple oversampling data augmentation method and show that it could further improve the performance. Finally, we demonstrate that our proposed model outperforms manual classification by neuroradiologists according to most performance metrics.
Human-Expert-Level Brain Tumor Detection Using Deep Learning with Data Distillation and Augmentation
Jul 16, 2020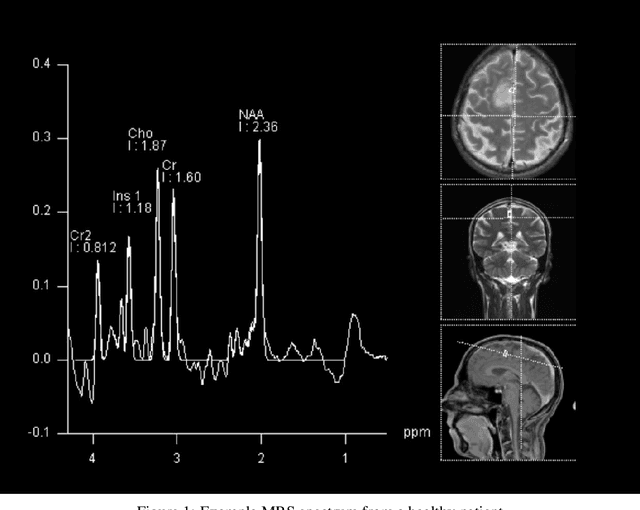
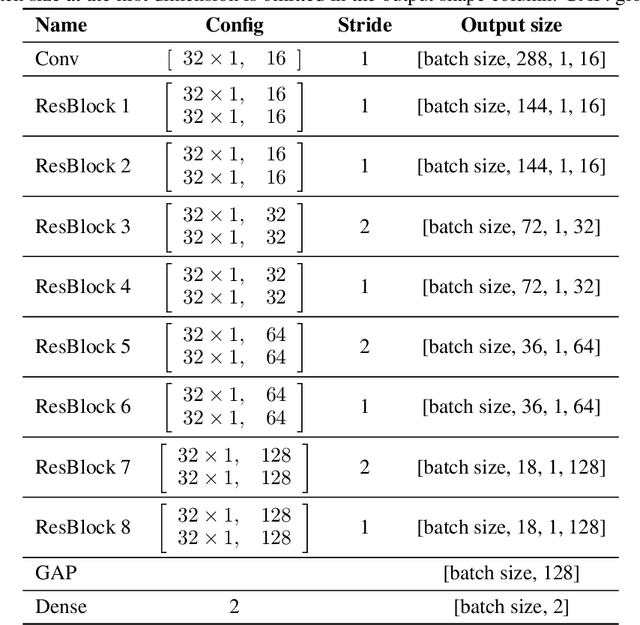
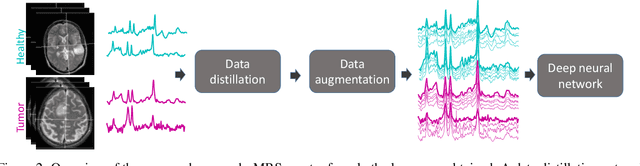
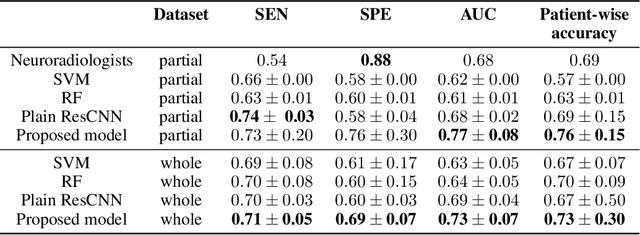
Abstract:The application of Deep Learning (DL) for medical diagnosis is often hampered by two problems. First, the amount of training data may be scarce, as it is limited by the number of patients who have acquired the condition to be diagnosed. Second, the training data may be corrupted by various types of noise. Here, we study the problem of brain tumor detection from magnetic resonance spectroscopy (MRS) data, where both types of problems are prominent. To overcome these challenges, we propose a new method for training a deep neural network that distills particularly representative training examples and augments the training data by mixing these samples from one class with those from the same and other classes to create additional training samples. We demonstrate that this technique substantially improves performance, allowing our method to reach human-expert-level accuracy with just a few thousand training examples. Interestingly, the network learns to rely on features of the data that are usually ignored by human experts, suggesting new directions for future research.
Staging Epileptogenesis with Deep Neural Networks
Jun 17, 2020



Abstract:Epilepsy is a common neurological disorder characterized by recurrent seizures accompanied by excessive synchronous brain activity. The process of structural and functional brain alterations leading to increased seizure susceptibility and eventually spontaneous seizures is called epileptogenesis (EPG) and can span months or even years. Detecting and monitoring the progression of EPG could allow for targeted early interventions that could slow down disease progression or even halt its development. Here, we propose an approach for staging EPG using deep neural networks and identify potential electroencephalography (EEG) biomarkers to distinguish different phases of EPG. Specifically, continuous intracranial EEG recordings were collected from a rodent model where epilepsy is induced by electrical perforant pathway stimulation (PPS). A deep neural network (DNN) is trained to distinguish EEG signals from before stimulation (baseline), shortly after the PPS and long after the PPS but before the first spontaneous seizure (FSS). Experimental results show that our proposed method can classify EEG signals from the three phases with an average area under the curve (AUC) of 0.93, 0.89, and 0.86. To the best of our knowledge, this represents the first successful attempt to stage EPG prior to the FSS using DNNs.
Towards Early Diagnosis of Epilepsy from EEG Data
Jun 17, 2020



Abstract:Epilepsy is one of the most common neurological disorders, affecting about 1% of the population at all ages. Detecting the development of epilepsy, i.e., epileptogenesis (EPG), before any seizures occur could allow for early interventions and potentially more effective treatments. Here, we investigate if modern machine learning (ML) techniques can detect EPG from intra-cranial electroencephalography (EEG) recordings prior to the occurrence of any seizures. For this we use a rodent model of epilepsy where EPG is triggered by electrical stimulation of the brain. We propose a ML framework for EPG identification, which combines a deep convolutional neural network (CNN) with a prediction aggregation method to obtain the final classification decision. Specifically, the neural network is trained to distinguish five second segments of EEG recordings taken from either the pre-stimulation period or the post-stimulation period. Due to the gradual development of epilepsy, there is enormous overlap of the EEG patterns before and after the stimulation. Hence, a prediction aggregation process is introduced, which pools predictions over a longer period. By aggregating predictions over one hour, our approach achieves an area under the curve (AUC) of 0.99 on the EPG detection task. This demonstrates the feasibility of EPG prediction from EEG recordings.
Residual Deep Convolutional Neural Network for EEG Signal Classification in Epilepsy
Mar 19, 2019



Abstract:Epilepsy is the fourth most common neurological disorder, affecting about 1% of the population at all ages. As many as 60% of people with epilepsy experience focal seizures which originate in a certain brain area and are limited to part of one cerebral hemisphere. In focal epilepsy patients, a precise surgical removal of the seizure onset zone can lead to effective seizure control or even a seizure-free outcome. Thus, correct identification of the seizure onset zone is essential. For clinical evaluation purposes, electroencephalography (EEG) recordings are commonly used. However, their interpretation is usually done manually by physicians and is time-consuming and error-prone. In this work, we propose an automated epileptic signal classification method based on modern deep learning methods. In contrast to previous approaches, the network is trained directly on the EEG recordings, avoiding hand-crafted feature extraction and selection procedures. This exploits the ability of deep neural networks to detect and extract relevant features automatically, that may be too complex or subtle to be noticed by humans. The proposed network structure is based on a convolutional neural network with residual connections. We demonstrate that our network produces state-of-the-art performance on two benchmark data sets, a data set from Bonn University and the Bern-Barcelona data set. We conclude that modern deep learning approaches can reach state-of-the-art performance on epileptic EEG classification and automated seizure onset zone identification tasks when trained on raw EEG data. This suggests that such approaches have potential for improving clinical practice.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge