Sean Grullon
Interpretability analysis on a pathology foundation model reveals biologically relevant embeddings across modalities
Jul 15, 2024Abstract:Mechanistic interpretability has been explored in detail for large language models (LLMs). For the first time, we provide a preliminary investigation with similar interpretability methods for medical imaging. Specifically, we analyze the features from a ViT-Small encoder obtained from a pathology Foundation Model via application to two datasets: one dataset of pathology images, and one dataset of pathology images paired with spatial transcriptomics. We discover an interpretable representation of cell and tissue morphology, along with gene expression within the model embedding space. Our work paves the way for further exploration around interpretable feature dimensions and their utility for medical and clinical applications.
PLUTO: Pathology-Universal Transformer
May 13, 2024



Abstract:Pathology is the study of microscopic inspection of tissue, and a pathology diagnosis is often the medical gold standard to diagnose disease. Pathology images provide a unique challenge for computer-vision-based analysis: a single pathology Whole Slide Image (WSI) is gigapixel-sized and often contains hundreds of thousands to millions of objects of interest across multiple resolutions. In this work, we propose PathoLogy Universal TransfOrmer (PLUTO): a light-weight pathology FM that is pre-trained on a diverse dataset of 195 million image tiles collected from multiple sites and extracts meaningful representations across multiple WSI scales that enable a large variety of downstream pathology tasks. In particular, we design task-specific adaptation heads that utilize PLUTO's output embeddings for tasks which span pathology scales ranging from subcellular to slide-scale, including instance segmentation, tile classification, and slide-level prediction. We compare PLUTO's performance to other state-of-the-art methods on a diverse set of external and internal benchmarks covering multiple biologically relevant tasks, tissue types, resolutions, stains, and scanners. We find that PLUTO matches or outperforms existing task-specific baselines and pathology-specific foundation models, some of which use orders-of-magnitude larger datasets and model sizes when compared to PLUTO. Our findings present a path towards a universal embedding to power pathology image analysis, and motivate further exploration around pathology foundation models in terms of data diversity, architectural improvements, sample efficiency, and practical deployability in real-world applications.
Using Whole Slide Image Representations from Self-Supervised Contrastive Learning for Melanoma Concordance Regression
Oct 10, 2022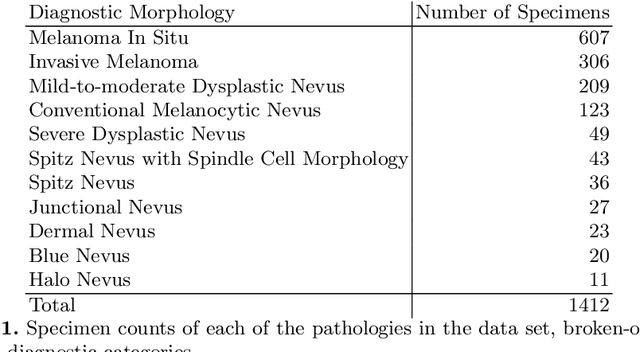
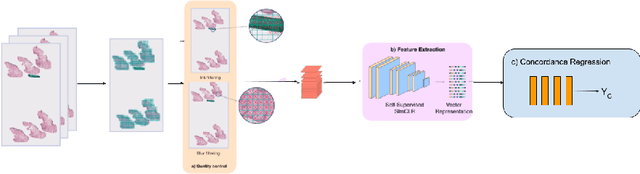
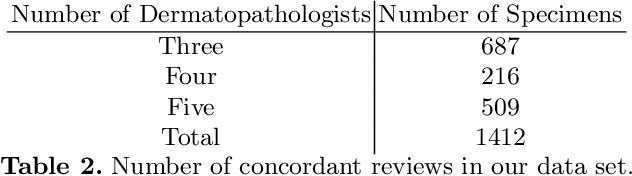
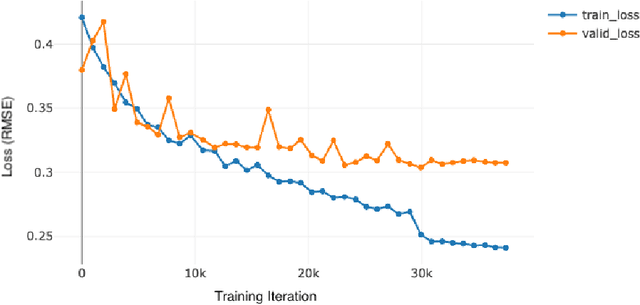
Abstract:Although melanoma occurs more rarely than several other skin cancers, patients' long term survival rate is extremely low if the diagnosis is missed. Diagnosis is complicated by a high discordance rate among pathologists when distinguishing between melanoma and benign melanocytic lesions. A tool that provides potential concordance information to healthcare providers could help inform diagnostic, prognostic, and therapeutic decision-making for challenging melanoma cases. We present a melanoma concordance regression deep learning model capable of predicting the concordance rate of invasive melanoma or melanoma in-situ from digitized Whole Slide Images (WSIs). The salient features corresponding to melanoma concordance were learned in a self-supervised manner with the contrastive learning method, SimCLR. We trained a SimCLR feature extractor with 83,356 WSI tiles randomly sampled from 10,895 specimens originating from four distinct pathology labs. We trained a separate melanoma concordance regression model on 990 specimens with available concordance ground truth annotations from three pathology labs and tested the model on 211 specimens. We achieved a Root Mean Squared Error (RMSE) of 0.28 +/- 0.01 on the test set. We also investigated the performance of using the predicted concordance rate as a malignancy classifier, and achieved a precision and recall of 0.85 +/- 0.05 and 0.61 +/- 0.06, respectively, on the test set. These results are an important first step for building an artificial intelligence (AI) system capable of predicting the results of consulting a panel of experts and delivering a score based on the degree to which the experts would agree on a particular diagnosis. Such a system could be used to suggest additional testing or other action such as ordering additional stains or genetic tests.
A Pathology Deep Learning System Capable of Triage of Melanoma Specimens Utilizing Dermatopathologist Consensus as Ground Truth
Sep 15, 2021



Abstract:Although melanoma occurs more rarely than several other skin cancers, patients' long term survival rate is extremely low if the diagnosis is missed. Diagnosis is complicated by a high discordance rate among pathologists when distinguishing between melanoma and benign melanocytic lesions. A tool that allows pathology labs to sort and prioritize melanoma cases in their workflow could improve turnaround time by prioritizing challenging cases and routing them directly to the appropriate subspecialist. We present a pathology deep learning system (PDLS) that performs hierarchical classification of digitized whole slide image (WSI) specimens into six classes defined by their morphological characteristics, including classification of "Melanocytic Suspect" specimens likely representing melanoma or severe dysplastic nevi. We trained the system on 7,685 images from a single lab (the reference lab), including the the largest set of triple-concordant melanocytic specimens compiled to date, and tested the system on 5,099 images from two distinct validation labs. We achieved Area Underneath the ROC Curve (AUC) values of 0.93 classifying Melanocytic Suspect specimens on the reference lab, 0.95 on the first validation lab, and 0.82 on the second validation lab. We demonstrate that the PDLS is capable of automatically sorting and triaging skin specimens with high sensitivity to Melanocytic Suspect cases and that a pathologist would only need between 30% and 60% of the caseload to address all melanoma specimens.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge