Sanjeev Grampurohit
PolyIE: A Dataset of Information Extraction from Polymer Material Scientific Literature
Nov 13, 2023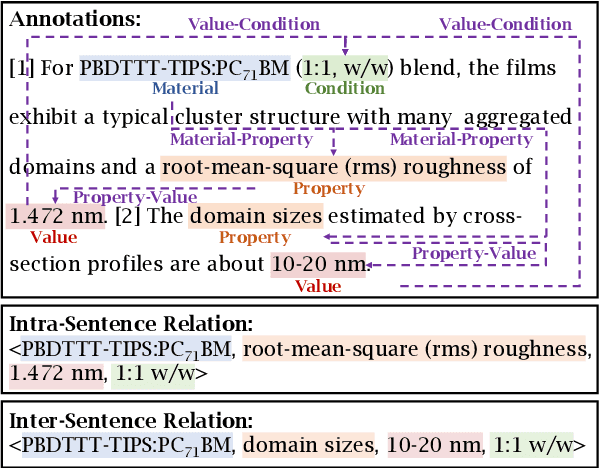



Abstract:Scientific information extraction (SciIE), which aims to automatically extract information from scientific literature, is becoming more important than ever. However, there are no existing SciIE datasets for polymer materials, which is an important class of materials used ubiquitously in our daily lives. To bridge this gap, we introduce POLYIE, a new SciIE dataset for polymer materials. POLYIE is curated from 146 full-length polymer scholarly articles, which are annotated with different named entities (i.e., materials, properties, values, conditions) as well as their N-ary relations by domain experts. POLYIE presents several unique challenges due to diverse lexical formats of entities, ambiguity between entities, and variable-length relations. We evaluate state-of-the-art named entity extraction and relation extraction models on POLYIE, analyze their strengths and weaknesses, and highlight some difficult cases for these models. To the best of our knowledge, POLYIE is the first SciIE benchmark for polymer materials, and we hope it will lead to more research efforts from the community on this challenging task. Our code and data are available on: https://github.com/jerry3027/PolyIE.
Brain Tumor Segmentation and Survival Prediction using Automatic Hard mining in 3D CNN Architecture
Jan 05, 2021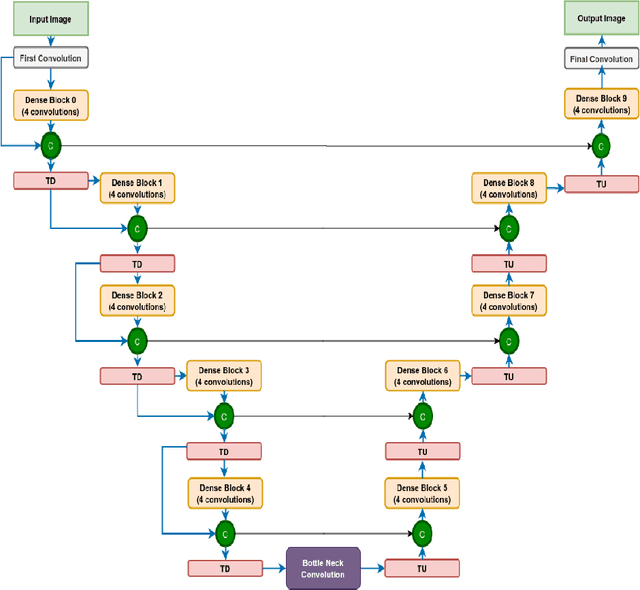
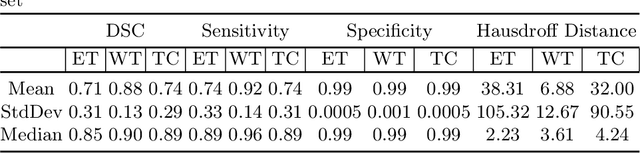
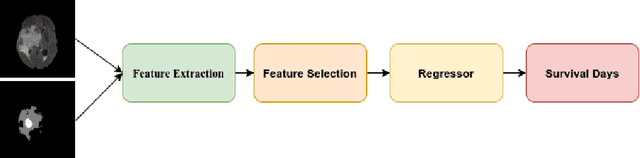
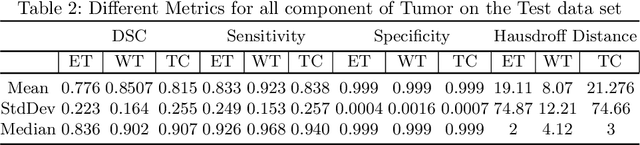
Abstract:We utilize 3-D fully convolutional neural networks (CNN) to segment gliomas and its constituents from multimodal Magnetic Resonance Images (MRI). The architecture uses dense connectivity patterns to reduce the number of weights and residual connections and is initialized with weights obtained from training this model with BraTS 2018 dataset. Hard mining is done during training to train for the difficult cases of segmentation tasks by increasing the dice similarity coefficient (DSC) threshold to choose the hard cases as epoch increases. On the BraTS2020 validation data (n = 125), this architecture achieved a tumor core, whole tumor, and active tumor dice of 0.744, 0.876, 0.714,respectively. On the test dataset, we get an increment in DSC of tumor core and active tumor by approximately 7%. In terms of DSC, our network performances on the BraTS 2020 test data are 0.775, 0.815, and 0.85 for enhancing tumor, tumor core, and whole tumor, respectively. Overall survival of a subject is determined using conventional machine learning from rediomics features obtained using a generated segmentation mask. Our approach has achieved 0.448 and 0.452 as the accuracy on the validation and test dataset.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge