Roy H Campbell
Model-Based Reinforcement Learning for Atari
Mar 05, 2019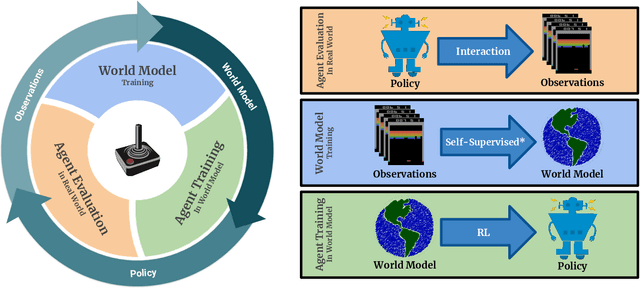
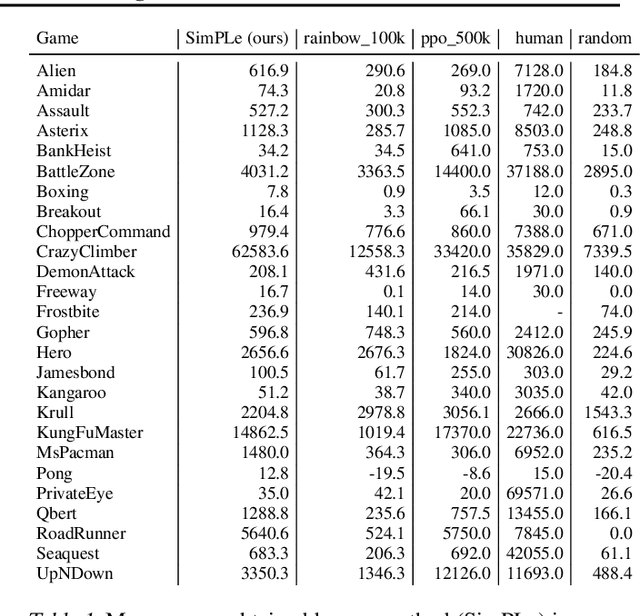
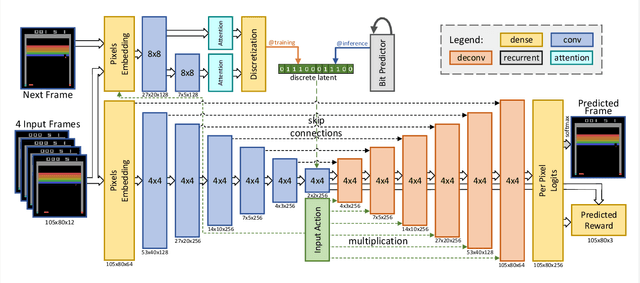
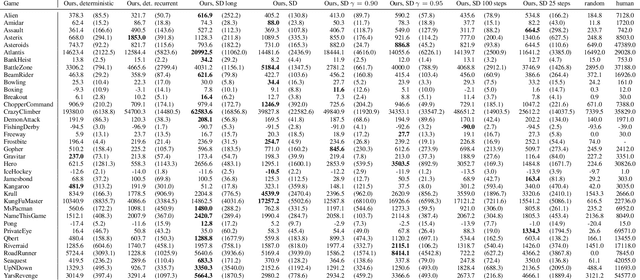
Abstract:Model-free reinforcement learning (RL) can be used to learn effective policies for complex tasks, such as Atari games, even from image observations. However, this typically requires very large amounts of interaction -- substantially more, in fact, than a human would need to learn the same games. How can people learn so quickly? Part of the answer may be that people can learn how the game works and predict which actions will lead to desirable outcomes. In this paper, we explore how video prediction models can similarly enable agents to solve Atari games with orders of magnitude fewer interactions than model-free methods. We describe Simulated Policy Learning (SimPLe), a complete model-based deep RL algorithm based on video prediction models and present a comparison of several model architectures, including a novel architecture that yields the best results in our setting. Our experiments evaluate SimPLe on a range of Atari games and achieve competitive results with only 100K interactions between the agent and the environment (400K frames), which corresponds to about two hours of real-time play.
Learning the progression and clinical subtypes of Alzheimer's disease from longitudinal clinical data
Dec 06, 2018

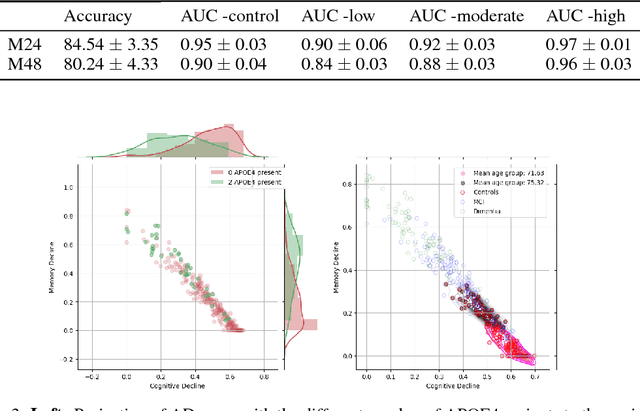

Abstract:Alzheimer's disease (AD) is a degenerative brain disease impairing a person's ability to perform day to day activities. The clinical manifestations of Alzheimer's disease are characterized by heterogeneity in age, disease span, progression rate, impairment of memory and cognitive abilities. Due to these variabilities, personalized care and treatment planning, as well as patient counseling about their individual progression is limited. Recent developments in machine learning to detect hidden patterns in complex, multi-dimensional datasets provides significant opportunities to address this critical need. In this work, we use unsupervised and supervised machine learning approaches for subtype identification and prediction. We apply machine learning methods to the extensive clinical observations available at the Alzheimer's Disease Neuroimaging Initiative (ADNI) data set to identify patient subtypes and to predict disease progression. Our analysis depicts the progression space for the Alzheimer's disease into low, moderate and high disease progression zones. The proposed work will enable early detection and characterization of distinct disease subtypes based on clinical heterogeneity. We anticipate that our models will enable patient counseling, clinical trial design, and ultimately individualized clinical care.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge