Vipul Satone
Fund2Vec: Mutual Funds Similarity using Graph Learning
Jun 24, 2021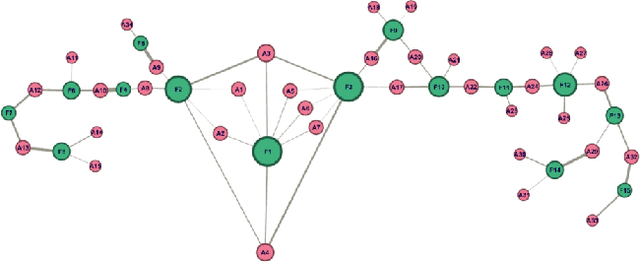
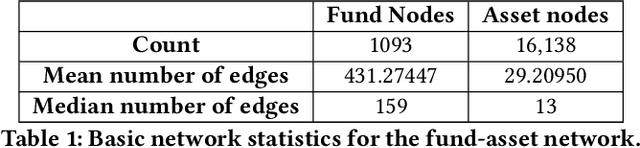
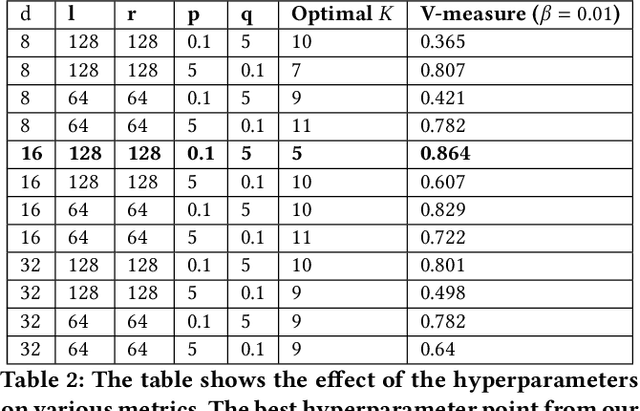
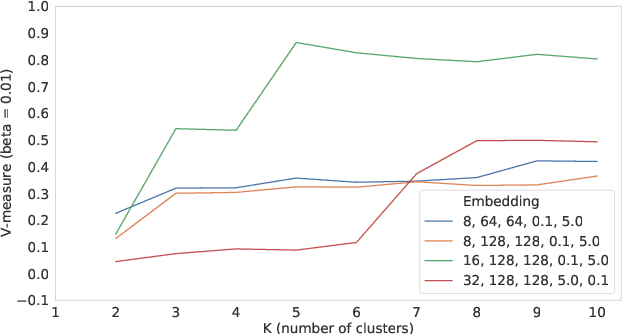
Abstract:Identifying similar mutual funds with respect to the underlying portfolios has found many applications in financial services ranging from fund recommender systems, competitors analysis, portfolio analytics, marketing and sales, etc. The traditional methods are either qualitative, and hence prone to biases and often not reproducible, or, are known not to capture all the nuances (non-linearities) among the portfolios from the raw data. We propose a radically new approach to identify similar funds based on the weighted bipartite network representation of funds and their underlying assets data using a sophisticated machine learning method called Node2Vec which learns an embedded low-dimensional representation of the network. We call the embedding \emph{Fund2Vec}. Ours is the first ever study of the weighted bipartite network representation of the funds-assets network in its original form that identifies structural similarity among portfolios as opposed to merely portfolio overlaps.
Learning the progression and clinical subtypes of Alzheimer's disease from longitudinal clinical data
Dec 06, 2018

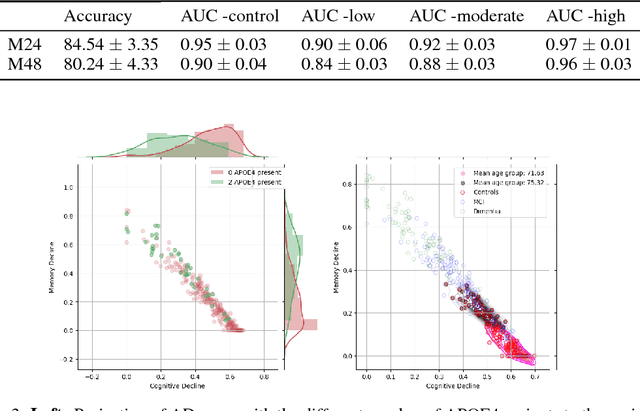

Abstract:Alzheimer's disease (AD) is a degenerative brain disease impairing a person's ability to perform day to day activities. The clinical manifestations of Alzheimer's disease are characterized by heterogeneity in age, disease span, progression rate, impairment of memory and cognitive abilities. Due to these variabilities, personalized care and treatment planning, as well as patient counseling about their individual progression is limited. Recent developments in machine learning to detect hidden patterns in complex, multi-dimensional datasets provides significant opportunities to address this critical need. In this work, we use unsupervised and supervised machine learning approaches for subtype identification and prediction. We apply machine learning methods to the extensive clinical observations available at the Alzheimer's Disease Neuroimaging Initiative (ADNI) data set to identify patient subtypes and to predict disease progression. Our analysis depicts the progression space for the Alzheimer's disease into low, moderate and high disease progression zones. The proposed work will enable early detection and characterization of distinct disease subtypes based on clinical heterogeneity. We anticipate that our models will enable patient counseling, clinical trial design, and ultimately individualized clinical care.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge