Qirong Li
Towards Universal Learning-based Model for Cardiac Image Reconstruction: Summary of the CMRxRecon2024 Challenge
Mar 05, 2025Abstract:Cardiovascular magnetic resonance (CMR) offers diverse imaging contrasts for assessment of cardiac function and tissue characterization. However, acquiring each single CMR modality is often time-consuming, and comprehensive clinical protocols require multiple modalities with various sampling patterns, further extending the overall acquisition time and increasing susceptibility to motion artifacts. Existing deep learning-based reconstruction methods are often designed for specific acquisition parameters, which limits their ability to generalize across a variety of scan scenarios. As part of the CMRxRecon Series, the CMRxRecon2024 challenge provides diverse datasets encompassing multi-modality multi-view imaging with various sampling patterns, and a platform for the international community to develop and benchmark reconstruction solutions in two well-crafted tasks. Task 1 is a modality-universal setting, evaluating the out-of-distribution generalization of the reconstructed model, while Task 2 follows sampling-universal setting assessing the one-for-all adaptability of the universal model. Main contributions include providing the first and largest publicly available multi-modality, multi-view cardiac k-space dataset; developing a benchmarking platform that simulates clinical acceleration protocols, with a shared code library and tutorial for various k-t undersampling patterns and data processing; giving technical insights of enhanced data consistency based on physic-informed networks and adaptive prompt-learning embedding to be versatile to different clinical settings; additional finding on evaluation metrics to address the limitations of conventional ground-truth references in universal reconstruction tasks.
CMRxRecon2024: A Multi-Modality, Multi-View K-Space Dataset Boosting Universal Machine Learning for Accelerated Cardiac MRI
Jun 27, 2024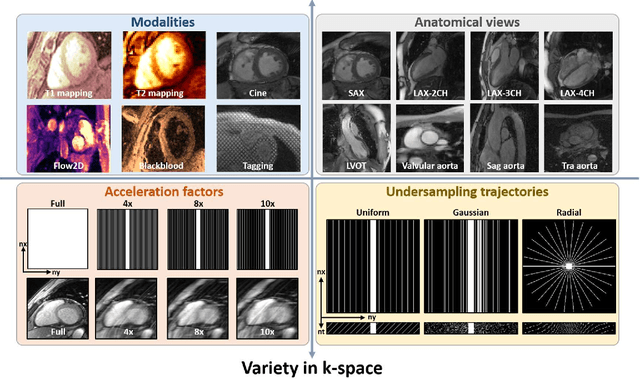
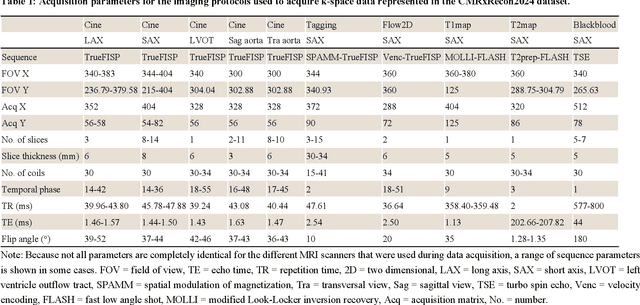
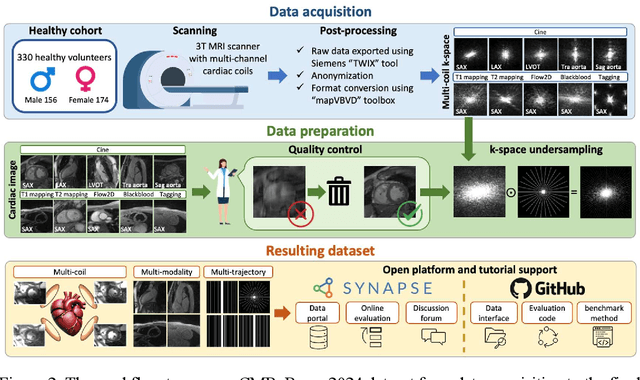
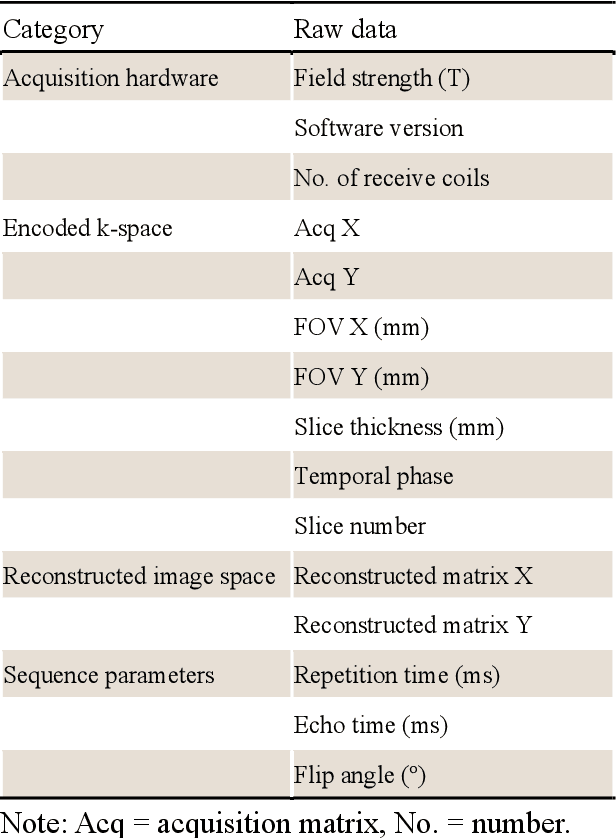
Abstract:Cardiac magnetic resonance imaging (MRI) has emerged as a clinically gold-standard technique for diagnosing cardiac diseases, thanks to its ability to provide diverse information with multiple modalities and anatomical views. Accelerated cardiac MRI is highly expected to achieve time-efficient and patient-friendly imaging, and then advanced image reconstruction approaches are required to recover high-quality, clinically interpretable images from undersampled measurements. However, the lack of publicly available cardiac MRI k-space dataset in terms of both quantity and diversity has severely hindered substantial technological progress, particularly for data-driven artificial intelligence. Here, we provide a standardized, diverse, and high-quality CMRxRecon2024 dataset to facilitate the technical development, fair evaluation, and clinical transfer of cardiac MRI reconstruction approaches, towards promoting the universal frameworks that enable fast and robust reconstructions across different cardiac MRI protocols in clinical practice. To the best of our knowledge, the CMRxRecon2024 dataset is the largest and most diverse publicly available cardiac k-space dataset. It is acquired from 330 healthy volunteers, covering commonly used modalities, anatomical views, and acquisition trajectories in clinical cardiac MRI workflows. Besides, an open platform with tutorials, benchmarks, and data processing tools is provided to facilitate data usage, advanced method development, and fair performance evaluation.
An Empirical Study on the Fairness of Foundation Models for Multi-Organ Image Segmentation
Jun 18, 2024



Abstract:The segmentation foundation model, e.g., Segment Anything Model (SAM), has attracted increasing interest in the medical image community. Early pioneering studies primarily concentrated on assessing and improving SAM's performance from the perspectives of overall accuracy and efficiency, yet little attention was given to the fairness considerations. This oversight raises questions about the potential for performance biases that could mirror those found in task-specific deep learning models like nnU-Net. In this paper, we explored the fairness dilemma concerning large segmentation foundation models. We prospectively curate a benchmark dataset of 3D MRI and CT scans of the organs including liver, kidney, spleen, lung and aorta from a total of 1056 healthy subjects with expert segmentations. Crucially, we document demographic details such as gender, age, and body mass index (BMI) for each subject to facilitate a nuanced fairness analysis. We test state-of-the-art foundation models for medical image segmentation, including the original SAM, medical SAM and SAT models, to evaluate segmentation efficacy across different demographic groups and identify disparities. Our comprehensive analysis, which accounts for various confounding factors, reveals significant fairness concerns within these foundational models. Moreover, our findings highlight not only disparities in overall segmentation metrics, such as the Dice Similarity Coefficient but also significant variations in the spatial distribution of segmentation errors, offering empirical evidence of the nuanced challenges in ensuring fairness in medical image segmentation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge