Priyanshu Sinha
Was there COVID-19 back in 2012? Challenge for AI in Diagnosis with Similar Indications
Jun 23, 2020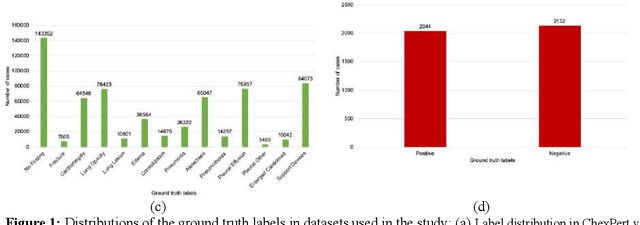
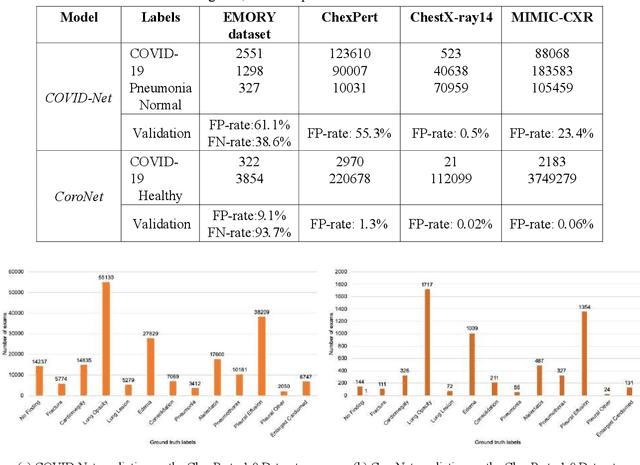
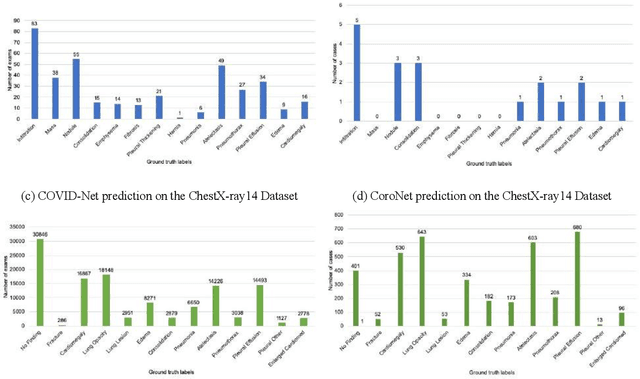

Abstract:Purpose: Since the recent COVID-19 outbreak, there has been an avalanche of research papers applying deep learning based image processing to chest radiographs for detection of the disease. To test the performance of the two top models for CXR COVID-19 diagnosis on external datasets to assess model generalizability. Methods: In this paper, we present our argument regarding the efficiency and applicability of existing deep learning models for COVID-19 diagnosis. We provide results from two popular models - COVID-Net and CoroNet evaluated on three publicly available datasets and an additional institutional dataset collected from EMORY Hospital between January and May 2020, containing patients tested for COVID-19 infection using RT-PCR. Results: There is a large false positive rate (FPR) for COVID-Net on both ChexPert (55.3%) and MIMIC-CXR (23.4%) dataset. On the EMORY Dataset, COVID-Net has 61.4% sensitivity, 0.54 F1-score and 0.49 precision value. The FPR of the CoroNet model is significantly lower across all the datasets as compared to COVID-Net - EMORY(9.1%), ChexPert (1.3%), ChestX-ray14 (0.02%), MIMIC-CXR (0.06%). Conclusion: The models reported good to excellent performance on their internal datasets, however we observed from our testing that their performance dramatically worsened on external data. This is likely from several causes including overfitting models due to lack of appropriate control patients and ground truth labels. The fourth institutional dataset was labeled using RT-PCR, which could be positive without radiographic findings and vice versa. Therefore, a fusion model of both clinical and radiographic data may have better performance and generalization.
Developing and Deploying Machine Learning Pipelines against Real-Time Image Streams from the PACS
Apr 20, 2020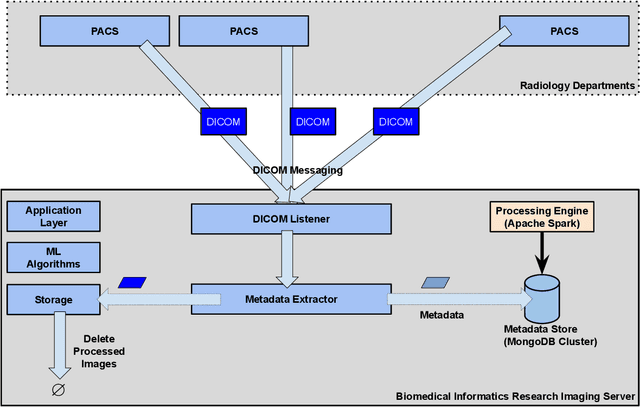
Abstract:Executing machine learning (ML) pipelines on radiology images is hard due to limited computing resources in clinical environments, whereas running them in research clusters in real-time requires efficient data transfer capabilities. We propose Niffler, an integrated ML framework that runs in research clusters that receives radiology images in real-time from hospitals' Picture Archiving and Communication Systems (PACS). Niffler consists of an inter-domain data streaming approach that exploits the Digital Imaging and Communications in Medicine (DICOM) protocol to fetch data from the PACS to the data processing servers for executing the ML pipelines. It provides metadata extraction capabilities and Application programming interfaces (APIs) to apply filters on the DICOM images and run the ML pipelines. The outcomes of the ML pipelines can then be shared back with the end-users in a de-identified manner. Evaluations on the Niffler prototype highlight the feasibility and efficiency in running the ML pipelines in real-time from a research cluster on the images received in real-time from hospital PACS.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge