Pi-Chuan Chang
Towards Generalist Biomedical AI
Jul 26, 2023



Abstract:Medicine is inherently multimodal, with rich data modalities spanning text, imaging, genomics, and more. Generalist biomedical artificial intelligence (AI) systems that flexibly encode, integrate, and interpret this data at scale can potentially enable impactful applications ranging from scientific discovery to care delivery. To enable the development of these models, we first curate MultiMedBench, a new multimodal biomedical benchmark. MultiMedBench encompasses 14 diverse tasks such as medical question answering, mammography and dermatology image interpretation, radiology report generation and summarization, and genomic variant calling. We then introduce Med-PaLM Multimodal (Med-PaLM M), our proof of concept for a generalist biomedical AI system. Med-PaLM M is a large multimodal generative model that flexibly encodes and interprets biomedical data including clinical language, imaging, and genomics with the same set of model weights. Med-PaLM M reaches performance competitive with or exceeding the state of the art on all MultiMedBench tasks, often surpassing specialist models by a wide margin. We also report examples of zero-shot generalization to novel medical concepts and tasks, positive transfer learning across tasks, and emergent zero-shot medical reasoning. To further probe the capabilities and limitations of Med-PaLM M, we conduct a radiologist evaluation of model-generated (and human) chest X-ray reports and observe encouraging performance across model scales. In a side-by-side ranking on 246 retrospective chest X-rays, clinicians express a pairwise preference for Med-PaLM M reports over those produced by radiologists in up to 40.50% of cases, suggesting potential clinical utility. While considerable work is needed to validate these models in real-world use cases, our results represent a milestone towards the development of generalist biomedical AI systems.
Knowledge distillation for fast and accurate DNA sequence correction
Nov 17, 2022



Abstract:Accurate genome sequencing can improve our understanding of biology and the genetic basis of disease. The standard approach for generating DNA sequences from PacBio instruments relies on HMM-based models. Here, we introduce Distilled DeepConsensus - a distilled transformer-encoder model for sequence correction, which improves upon the HMM-based methods with runtime constraints in mind. Distilled DeepConsensus is 1.3x faster and 1.5x smaller than its larger counterpart while improving the yield of high quality reads (Q30) over the HMM-based method by 1.69x (vs. 1.73x for larger model). With improved accuracy of genomic sequences, Distilled DeepConsensus improves downstream applications of genomic sequence analysis such as reducing variant calling errors by 39% (34% for larger model) and improving genome assembly quality by 3.8% (4.2% for larger model). We show that the representations learned by Distilled DeepConsensus are similar between faster and slower models.
Learning from Data-Rich Problems: A Case Study on Genetic Variant Calling
Nov 15, 2019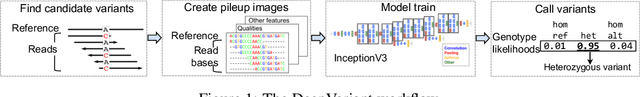
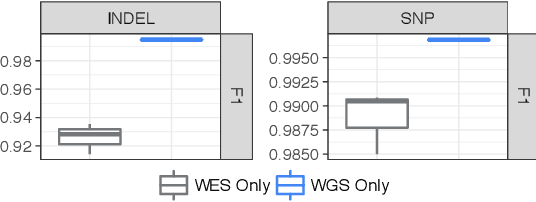

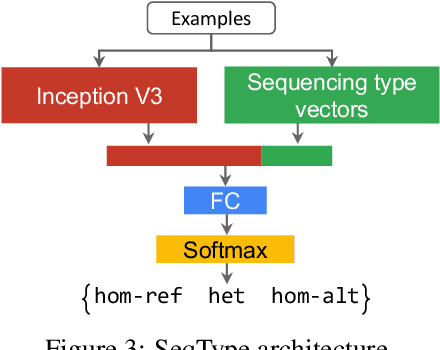
Abstract:Next Generation Sequencing can sample the whole genome (WGS) or the 1-2% of the genome that codes for proteins called the whole exome (WES). Machine learning approaches to variant calling achieve high accuracy in WGS data, but the reduced number of training examples causes training with WES data alone to achieve lower accuracy. We propose and compare three different data augmentation strategies for improving performance on WES data: 1) joint training with WES and WGS data, 2) warmstarting the WES model from a WGS model, and 3) joint training with the sequencing type specified. All three approaches show improved accuracy over a model trained using just WES data, suggesting the ability of models to generalize insights from the greater WGS data while retaining performance on the specialized WES problem. These data augmentation approaches may apply to other problem areas in genomics, where several specialized models would each see only a subset of the genome.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge