Philipp Sommer
Risk Classification of Brain Metastases via Radiomics, Delta-Radiomics and Machine Learning
Feb 17, 2023



Abstract:Stereotactic radiotherapy (SRT) is one of the most important treatment for patients with brain metastases (BM). Conventionally, following SRT patients are monitored by serial imaging and receive salvage treatments in case of significant tumor growth. We hypothesized that using radiomics and machine learning (ML), metastases at high risk for subsequent progression could be identified during follow-up prior to the onset of significant tumor growth, enabling personalized follow-up intervals and early selection for salvage treatment. All experiments are performed on a dataset from clinical routine of the Radiation Oncology department of the University Hospital Erlangen (UKER). The classification is realized via the maximum-relevance minimal-redundancy (MRMR) technique and support vector machines (SVM). The pipeline leads to a classification with a mean area under the curve (AUC) score of 0.83 in internal cross-validation and allows a division of the cohort into two subcohorts that differ significantly in their median time to progression (low-risk metastasis (LRM): 17.3 months, high-risk metastasis (HRM): 9.6 months, p < 0.01). The classification performance is especially enhanced by the analysis of medical images from different points in time (AUC 0.53 -> AUC 0.74). The results indicate that risk stratification of BM based on radiomics and machine learning during post-SRT follow-up is possible with good accuracy and should be further pursued to personalize and improve post-SRT follow-up.
Deep learning for brain metastasis detection and segmentation in longitudinal MRI data
Dec 28, 2021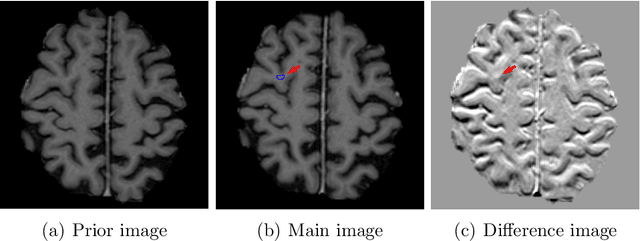
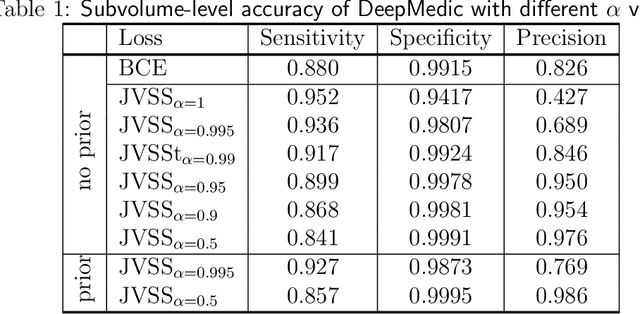
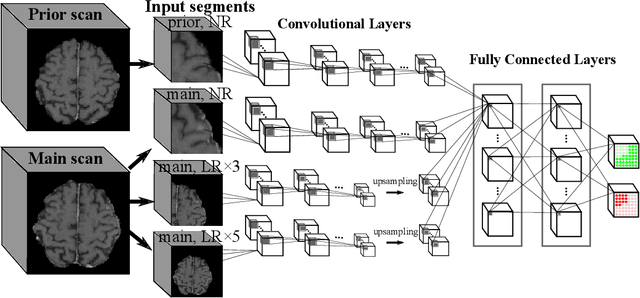
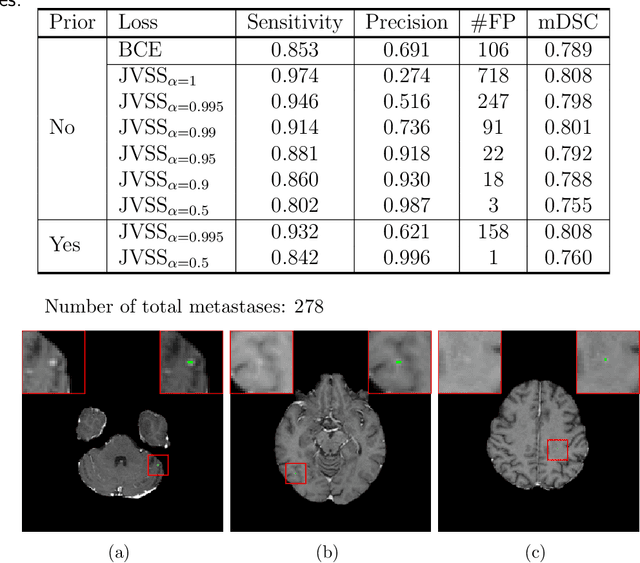
Abstract:Brain metastases occur frequently in patients with metastatic cancer. Early and accurate detection of brain metastases is very essential for treatment planning and prognosis in radiation therapy. To improve brain metastasis detection performance with deep learning, a custom detection loss called volume-level sensitivity-specificity (VSS) is proposed, which rates individual metastasis detection sensitivity and specificity in (sub-)volume levels. As sensitivity and precision are always a trade-off in a metastasis level, either a high sensitivity or a high precision can be achieved by adjusting the weights in the VSS loss without decline in dice score coefficient for segmented metastases. To reduce metastasis-like structures being detected as false positive metastases, a temporal prior volume is proposed as an additional input of the neural network. Our proposed VSS loss improves the sensitivity of brain metastasis detection, increasing the sensitivity from 86.7% to 95.5%. Alternatively, it improves the precision from 68.8% to 97.8%. With the additional temporal prior volume, about 45% of the false positive metastases are reduced in the high sensitivity model and the precision reaches 99.6% for the high specificity model. The mean dice coefficient for all metastases is about 0.81. With the ensemble of the high sensitivity and high specificity models, on average only 1.5 false positive metastases per patient needs further check, while the majority of true positive metastases are confirmed. The ensemble learning is able to distinguish high confidence true positive metastases from metastases candidates that require special expert review or further follow-up, being particularly well-fit to the requirements of expert support in real clinical practice.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge