Paula Ramirez Gilliland
Multi-Center Fetal Brain Tissue Annotation (FeTA) Challenge 2022 Results
Feb 08, 2024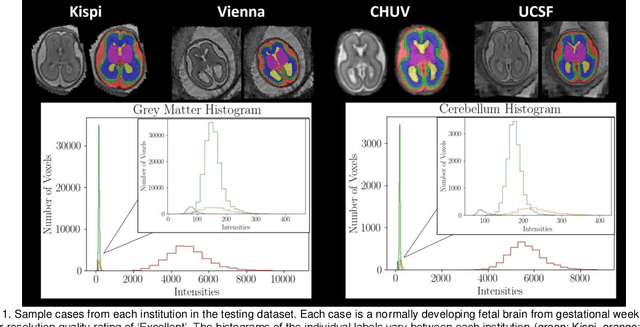
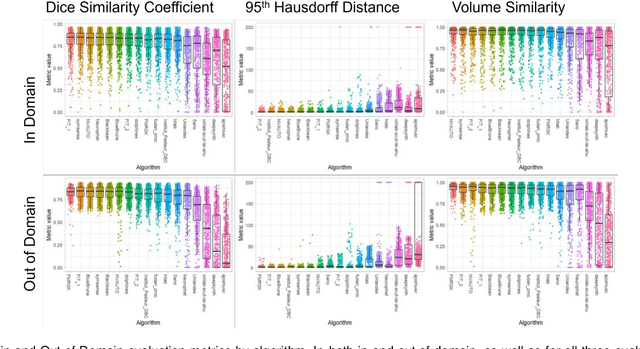
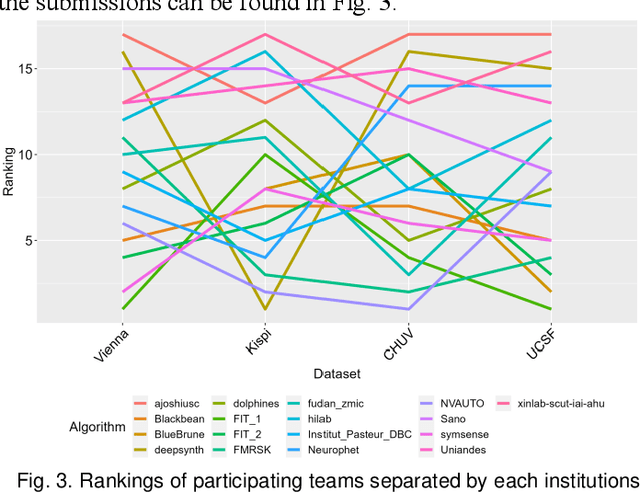
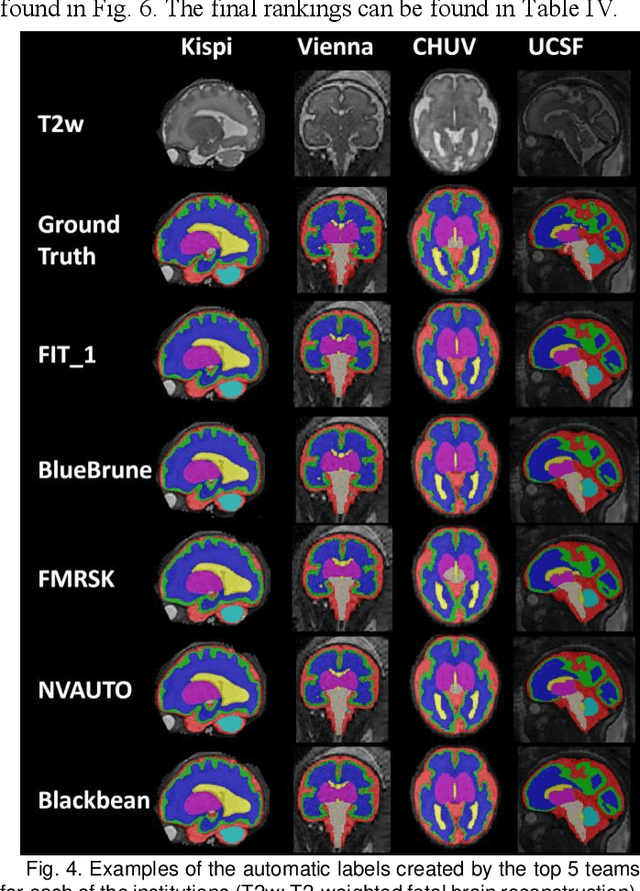
Abstract:Segmentation is a critical step in analyzing the developing human fetal brain. There have been vast improvements in automatic segmentation methods in the past several years, and the Fetal Brain Tissue Annotation (FeTA) Challenge 2021 helped to establish an excellent standard of fetal brain segmentation. However, FeTA 2021 was a single center study, and the generalizability of algorithms across different imaging centers remains unsolved, limiting real-world clinical applicability. The multi-center FeTA Challenge 2022 focuses on advancing the generalizability of fetal brain segmentation algorithms for magnetic resonance imaging (MRI). In FeTA 2022, the training dataset contained images and corresponding manually annotated multi-class labels from two imaging centers, and the testing data contained images from these two imaging centers as well as two additional unseen centers. The data from different centers varied in many aspects, including scanners used, imaging parameters, and fetal brain super-resolution algorithms applied. 16 teams participated in the challenge, and 17 algorithms were evaluated. Here, a detailed overview and analysis of the challenge results are provided, focusing on the generalizability of the submissions. Both in- and out of domain, the white matter and ventricles were segmented with the highest accuracy, while the most challenging structure remains the cerebral cortex due to anatomical complexity. The FeTA Challenge 2022 was able to successfully evaluate and advance generalizability of multi-class fetal brain tissue segmentation algorithms for MRI and it continues to benchmark new algorithms. The resulting new methods contribute to improving the analysis of brain development in utero.
PIPPI2021: An Approach to Automated Diagnosis and Texture Analysis of the Fetal Liver & Placenta in Fetal Growth Restriction
Nov 01, 2022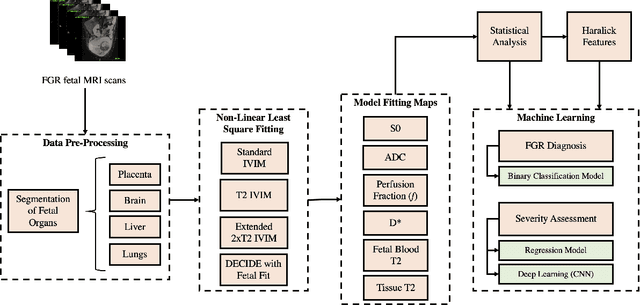
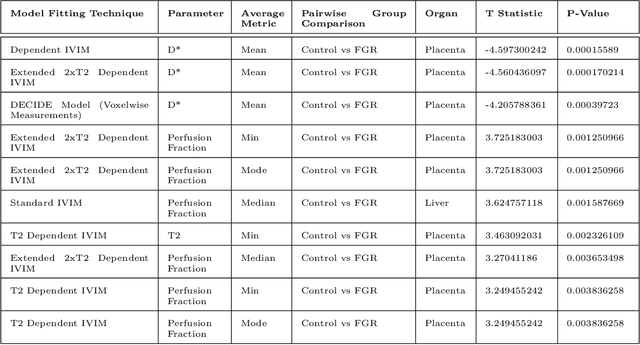
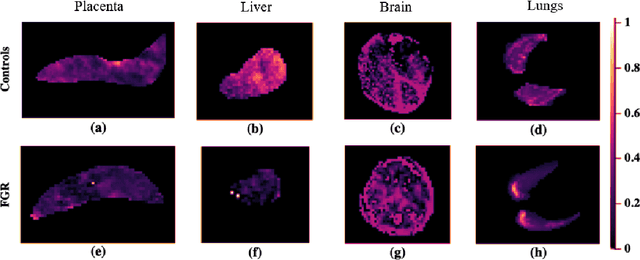
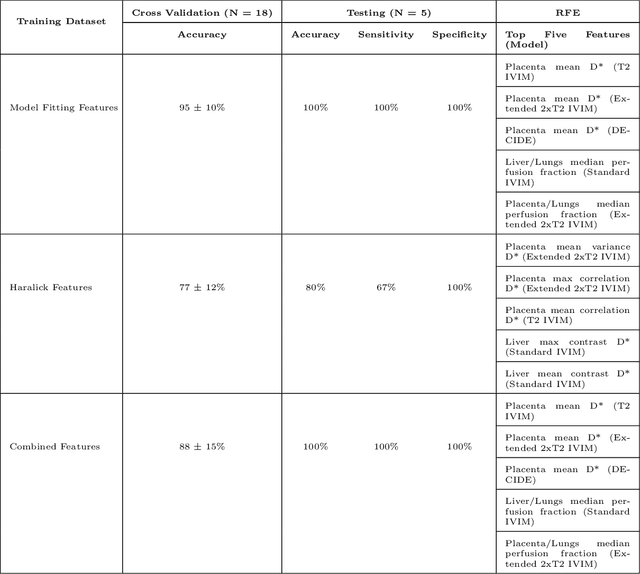
Abstract:Fetal growth restriction (FGR) is a prevalent pregnancy condition characterised by failure of the fetus to reach its genetically predetermined growth potential. We explore the application of model fitting techniques, linear regression machine learning models, deep learning regression, and Haralick textured features from multi-contrast MRI for multi-fetal organ analysis of FGR. We employed T2 relaxometry and diffusion-weighted MRI datasets (using a combined T2-diffusion scan) for 12 normally grown and 12 FGR gestational age (GA) matched pregnancies. We applied the Intravoxel Incoherent Motion Model and novel multi-compartment models for MRI fetal analysis, which exhibit potential to provide a multi-organ FGR assessment, overcoming the limitations of empirical indicators - such as abnormal artery Doppler findings - to evaluate placental dysfunction. The placenta and fetal liver presented key differentiators between FGR and normal controls (decreased perfusion, abnormal fetal blood motion and reduced fetal blood oxygenation. This may be associated with the preferential shunting of the fetal blood towards the fetal brain. These features were further explored to determine their role in assessing FGR severity, by employing simple machine learning models to predict FGR diagnosis (100\% accuracy in test data, n=5), GA at delivery, time from MRI scan to delivery, and baby weight. Moreover, we explored the use of deep learning to regress the latter three variables. Image texture analysis of the fetal organs demonstrated prominent textural variations in the placental perfusion fractions maps between the groups (p$<$0.0009), and spatial differences in the incoherent fetal capillary blood motion in the liver (p$<$0.009). This research serves as a proof-of-concept, investigating the effect of FGR on fetal organs.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge