Nigel H. Lovell
SemanticST: Spatially Informed Semantic Graph Learning for Clustering, Integration, and Scalable Analysis of Spatial Transcriptomics
Jun 16, 2025Abstract:Spatial transcriptomics (ST) technologies enable gene expression profiling with spatial resolution, offering unprecedented insights into tissue organization and disease heterogeneity. However, current analysis methods often struggle with noisy data, limited scalability, and inadequate modelling of complex cellular relationships. We present SemanticST, a biologically informed, graph-based deep learning framework that models diverse cellular contexts through multi-semantic graph construction. SemanticST builds multiple context-specific graphs capturing spatial proximity, gene expression similarity, and tissue domain structure, and learns disentangled embeddings for each. These are fused using an attention-inspired strategy to yield a unified, biologically meaningful representation. A community-aware min-cut loss improves robustness over contrastive learning, particularly in sparse ST data. SemanticST supports mini-batch training, making it the first graph neural network scalable to large-scale datasets such as Xenium (500,000 cells). Benchmarking across four platforms (Visium, Slide-seq, Stereo-seq, Xenium) and multiple human and mouse tissues shows consistent 20 percentage gains in ARI, NMI, and trajectory fidelity over DeepST, GraphST, and IRIS. In re-analysis of breast cancer Xenium data, SemanticST revealed rare and clinically significant niches, including triple receptor-positive clusters, spatially distinct DCIS-to-IDC transition zones, and FOXC2 tumour-associated myoepithelial cells, suggesting non-canonical EMT programs with stem-like features. SemanticST thus provides a scalable, interpretable, and biologically grounded framework for spatial transcriptomics analysis, enabling robust discovery across tissue types and diseases, and paving the way for spatially resolved tissue atlases and next-generation precision medicine.
Smart Textile-Driven Soft Spine Exosuit for Lifting Tasks in Industrial Applications
Feb 04, 2024Abstract:Work related musculoskeletal disorders (WMSDs) are often caused by repetitive lifting, making them a significant concern in occupational health. Although wearable assist devices have become the norm for mitigating the risk of back pain, most spinal assist devices still possess a partially rigid structure that impacts the user comfort and flexibility. This paper addresses this issue by presenting a smart textile actuated spine assistance robotic exosuit (SARE), which can conform to the back seamlessly without impeding the user movement and is incredibly lightweight. The SARE can assist the human erector spinae to complete any action with virtually infinite degrees of freedom. To detect the strain on the spine and to control the smart textile automatically, a soft knitting sensor which utilizes fluid pressure as sensing element is used. The new device is validated experimentally with human subjects where it reduces peak electromyography (EMG) signals of lumbar erector spinae by around 32 percent in loaded and around 22 percent in unloaded conditions. Moreover, the integrated EMG decreased by around 24.2 percent under loaded condition and around 23.6 percent under unloaded condition. In summary, the artificial muscle wearable device represents an anatomical solution to reduce the risk of muscle strain, metabolic energy cost and back pain associated with repetitive lifting tasks.
Fully Elman Neural Network: A Novel Deep Recurrent Neural Network Optimized by an Improved Harris Hawks Algorithm for Classification of Pulmonary Arterial Wedge Pressure
Jan 16, 2023



Abstract:Heart failure (HF) is one of the most prevalent life-threatening cardiovascular diseases in which 6.5 million people are suffering in the USA and more than 23 million worldwide. Mechanical circulatory support of HF patients can be achieved by implanting a left ventricular assist device (LVAD) into HF patients as a bridge to transplant, recovery or destination therapy and can be controlled by measurement of normal and abnormal pulmonary arterial wedge pressure (PAWP). While there are no commercial long-term implantable pressure sensors to measure PAWP, real-time non-invasive estimation of abnormal and normal PAWP becomes vital. In this work, first an improved Harris Hawks optimizer algorithm called HHO+ is presented and tested on 24 unimodal and multimodal benchmark functions. Second, a novel fully Elman neural network (FENN) is proposed to improve the classification performance. Finally, four novel 18-layer deep learning methods of convolutional neural networks (CNNs) with multi-layer perceptron (CNN-MLP), CNN with Elman neural networks (CNN-ENN), CNN with fully Elman neural networks (CNN-FENN), and CNN with fully Elman neural networks optimized by HHO+ algorithm (CNN-FENN-HHO+) for classification of abnormal and normal PAWP using estimated HVAD pump flow were developed and compared. The estimated pump flow was derived by a non-invasive method embedded into the commercial HVAD controller. The proposed methods are evaluated on an imbalanced clinical dataset using 5-fold cross-validation. The proposed CNN-FENN-HHO+ method outperforms the proposed CNN-MLP, CNN-ENN and CNN-FENN methods and improved the classification performance metrics across 5-fold cross-validation. The proposed methods can reduce the likelihood of hazardous events like pulmonary congestion and ventricular suction for HF patients and notify identified abnormal cases to the hospital, clinician and cardiologist.
A Sensorless Control System for an Implantable Heart Pump using a Real-time Deep Convolutional Neural Network
Apr 30, 2021



Abstract:Left ventricular assist devices (LVADs) are mechanical pumps, which can be used to support heart failure (HF) patients as bridge to transplant and destination therapy. To automatically adjust the LVAD speed, a physiological control system needs to be designed to respond to variations of patient hemodynamics across a variety of clinical scenarios. These control systems require pressure feedback signals from the cardiovascular system. However, there are no suitable long-term implantable sensors available. In this study, a novel real-time deep convolutional neural network (CNN) for estimation of preload based on the LVAD flow was proposed. A new sensorless adaptive physiological control system for an LVAD pump was developed using the full dynamic form of model free adaptive control (FFDL-MFAC) and the proposed preload estimator to maintain the patient conditions in safe physiological ranges. The CNN model for preload estimation was trained and evaluated through 10-fold cross validation on 100 different patient conditions and the proposed sensorless control system was assessed on a new testing set of 30 different patient conditions across six different patient scenarios. The proposed preload estimator was extremely accurate with a correlation coefficient of 0.97, root mean squared error of 0.84 mmHg, reproducibility coefficient of 1.56 mmHg, coefficient of variation of 14.44 %, and bias of 0.29 mmHg for the testing dataset. The results also indicate that the proposed sensorless physiological controller works similarly to the preload-based physiological control system for LVAD using measured preload to prevent ventricular suction and pulmonary congestion. This study shows that the LVADs can respond appropriately to changing patient states and physiological demands without the need for additional pressure or flow measurements.
Estimating Lower Body Kinematics using a Lie Group Constrained Extended Kalman Filter and Reduced IMU Count
Mar 21, 2021



Abstract:Goal: This paper presents an algorithm for estimating pelvis, thigh, shank, and foot kinematics during walking using only two or three wearable inertial sensors. Methods: The algorithm makes novel use of a Lie-group-based extended Kalman filter. The algorithm iterates through the prediction (kinematic equation), measurement (pelvis position pseudo-measurements, zero-velocity update, and flat-floor assumption), and constraint update (hinged knee and ankle joints, constant leg lengths). Results: The inertial motion capture algorithm was extensively evaluated on two datasets showing its performance against two standard benchmark approaches in optical motion capture (i.e., plug-in gait (commonly used in gait analysis) and a kinematic fit (commonly used in animation, robotics, and musculoskeleton simulation)), giving insight into the similarity and differences between the said approaches used in different application areas. The overall mean body segment position (relative to mid-pelvis origin) and orientation error magnitude of our algorithm ($n=14$ participants) for free walking was $5.93 \pm 1.33$ cm and $13.43 \pm 1.89^\circ$ when using three IMUs placed on the feet and pelvis, and $6.35 \pm 1.20$ cm and $12.71 \pm 1.60^\circ$ when using only two IMUs placed on the feet. Conclusion: The algorithm was able to track the joint angles in the sagittal plane for straight walking well, but requires improvement for unscripted movements (e.g., turning around, side steps), especially for dynamic movements or when considering clinical applications. Significance: This work has brought us closer to comprehensive remote gait monitoring using IMUs on the shoes. The low computational cost also suggests that it can be used in real-time with gait assistive devices.
Advanced Intelligent Systems for Surgical Robotics
Jan 02, 2020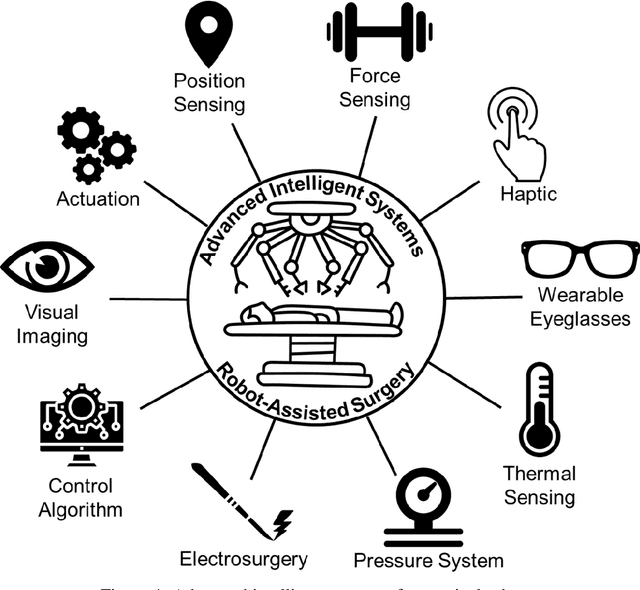
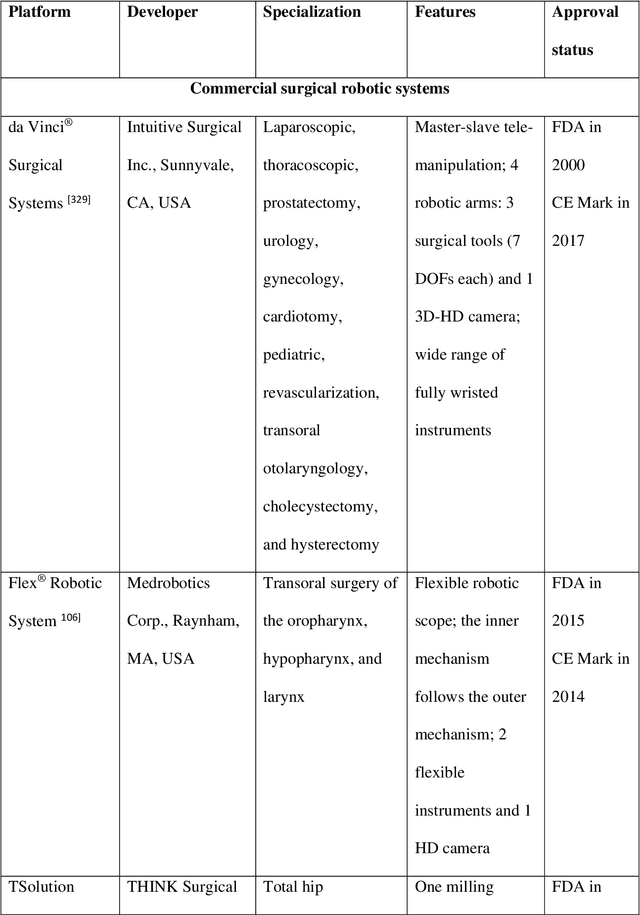
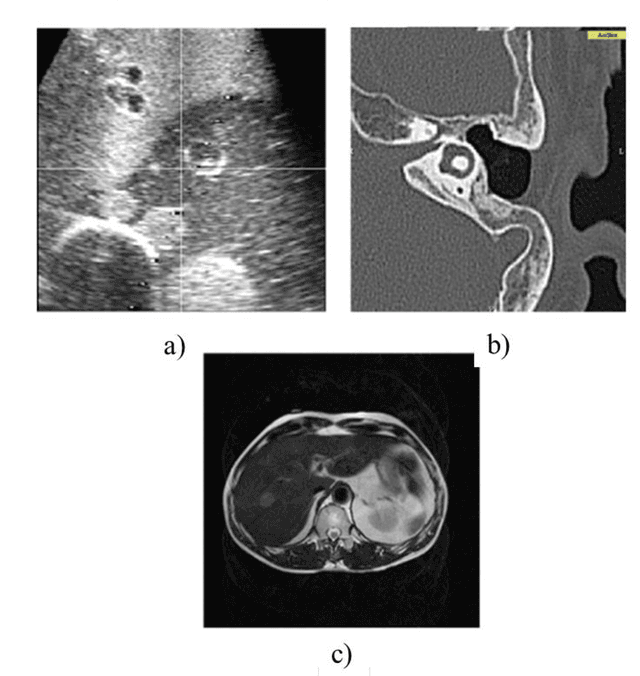
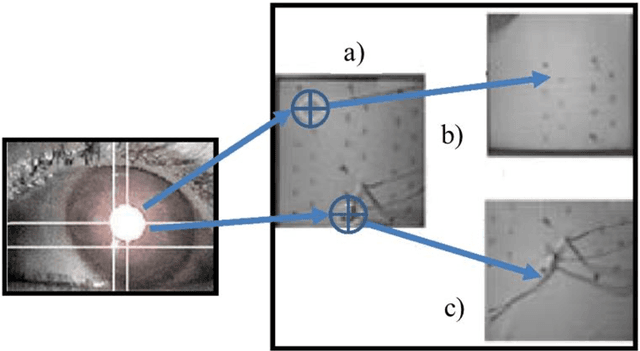
Abstract:Surgical robots have had clinical use since the mid 1990s. Robot-assisted surgeries offer many benefits over the conventional approach including lower risk of infection and blood loss, shorter recovery, and an overall safer procedure for patients. The past few decades have shown many emerging surgical robotic platforms that can work in complex and confined channels of the internal human organs and improve the cognitive and physical skills of the surgeons during the operation. Advanced technologies for sensing, actuation, and intelligent control have enabled multiple surgical devices to simultaneously operate within the human body at low cost and with more efficiency. Despite advances, current surgical intervention systems are not able to execute autonomous tasks and make cognitive decisions that are analogous to that of humans. This paper will overview a historical development of surgery from conventional open to robotic-assisted approaches with discussion on the capabilities of advanced intelligent systems and devices that are currently implemented in existing surgical robotic systems. It will also revisit available autonomous surgical platforms with comments on the essential technologies, existing challenges, and suggestions for the future development of intelligent robotic-assisted surgical systems towards the achievement of fully autonomous operation.
Estimating Lower Limb Kinematics using a Reduced Wearable Sensor Count
Oct 30, 2019



Abstract:Goal: This paper presents an algorithm for accurately estimating pelvis, thigh, and shank kinematics during walking using only three wearable inertial sensors. Methods: The algorithm makes novel use of a constrained Kalman filter (CKF). The algorithm iterates through the prediction (kinematic equation), measurement (pelvis position pseudo-measurements, zero velocity update, flat-floor assumption, and covariance limiter), and constraint update (formulation of hinged knee joints and ball-and-socket hip joints). Results: Evaluation of the algorithm using a Vicon-based sensor-to-segment calibration on nine participants ($7$ men and $2$ women, weight $63.0 \pm 6.8$ kg, height $1.70 \pm 0.06$ m, age $24.6 \pm 3.9$ years old), with no known gait or lower body biomechanical abnormalities, who walked within a $4 \times 4$ m$^2$ capture area shows that it can track motion relative to the mid-pelvis origin with mean position and orientation (no bias) root-mean-square error (RMSE) of $5.21 \pm 1.3$ cm and $16.1 \pm 3.2^\circ$, respectively. The sagittal knee and hip joint angle RMSEs (no bias) were $10.0 \pm 2.9^\circ$ and $9.9 \pm 3.2^\circ$, respectively, while the corresponding correlation coefficient (CC) values were $0.87 \pm 0.08$ and $0.74 \pm 0.12$. Conclusion: The CKF-based algorithm was able to track the 3D pose of the pelvis, thigh, and shanks using only three inertial sensors worn on the pelvis and shanks. Significance: Due to the Kalman-filter-based algorithm's low computation cost and the relative convenience of using only three wearable sensors, gait parameters can be computed in real-time and remotely for long-term gait monitoring. Furthermore, the system can be used to inform real-time gait assistive devices.
Estimating Lower Limb Kinematics using a Lie Group Constrained EKF and a Reduced Wearable IMU Count
Oct 04, 2019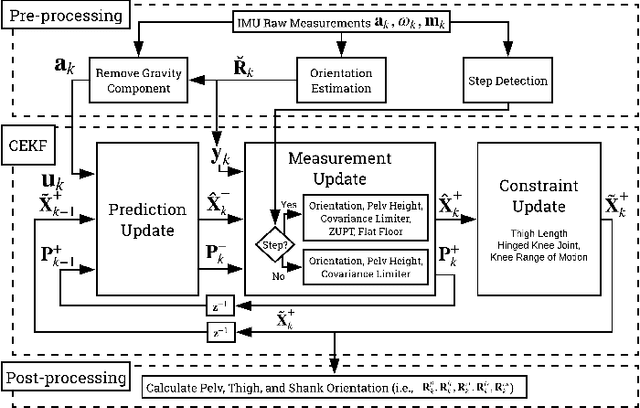
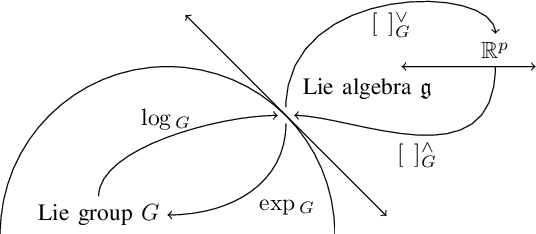
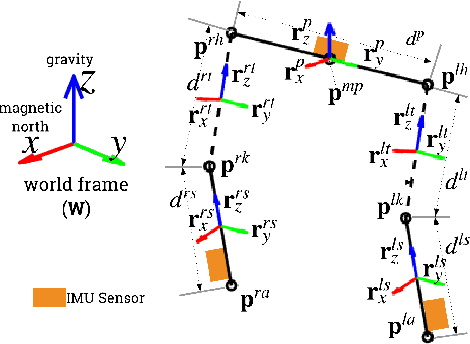
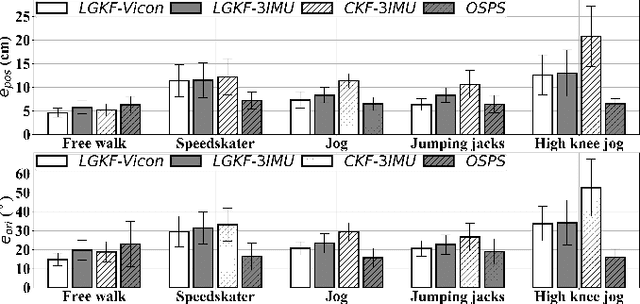
Abstract:This paper presents an algorithm that makes novel use of Lie group representation of position and orientation alongside a constrained extended Kalman filter (CEKF) for accurately estimating pelvis, thigh, and shank kinematics during walking using only three wearable inertial sensors. The algorithm iterates through the prediction update (kinematic equation), measurement update (pelvis height, zero velocity update, flat-floor assumption, and covariance limiter), and constraint update (formulation of hinged knee joints and ball-and-socket hip joints). Evaluation of the algorithm on nine healthy subjects who walked freely within a $4 \times 4$ m$^3$ room shows that it can track motion relative to the mid-pelvis origin with mean position and orientation root-mean-square error of $5.75 \pm 1.4$ cm and $19.8 \pm 5.2^\circ$, respectively. The sagittal knee and hip joint angle correlation coefficients were $0.88 \pm 0.1$ and $0.77 \pm 0.1$. This paper demonstrates an application of Lie group representation for inertial motion capture. Furthermore, the algorithm can compute gait parameters in real-time and, hence, can be used to inform gait assistive devices.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge