Neil Joshi
Unconstrained Large-scale 3D Reconstruction and Rendering across Altitudes
Apr 29, 2025Abstract:Production of photorealistic, navigable 3D site models requires a large volume of carefully collected images that are often unavailable to first responders for disaster relief or law enforcement. Real-world challenges include limited numbers of images, heterogeneous unposed cameras, inconsistent lighting, and extreme viewpoint differences for images collected from varying altitudes. To promote research aimed at addressing these challenges, we have developed the first public benchmark dataset for 3D reconstruction and novel view synthesis based on multiple calibrated ground-level, security-level, and airborne cameras. We present datasets that pose real-world challenges, independently evaluate calibration of unposed cameras and quality of novel rendered views, demonstrate baseline performance using recent state-of-practice methods, and identify challenges for further research.
AI Fairness via Domain Adaptation
Mar 15, 2021

Abstract:While deep learning (DL) approaches are reaching human-level performance for many tasks, including for diagnostics AI, the focus is now on challenges possibly affecting DL deployment, including AI privacy, domain generalization, and fairness. This last challenge is addressed in this study. Here we look at a novel method for ensuring AI fairness with respect to protected or sensitive factors. This method uses domain adaptation via training set enhancement to tackle bias-causing training data imbalance. More specifically, it uses generative models that allow the generation of more synthetic training samples for underrepresented populations. This paper applies this method to the use case of detection of age related macular degeneration (AMD). Our experiments show that starting with an originally biased AMD diagnostics model the method has the ability to improve fairness.
RENATA: REpreseNtation And Training Alteration for Bias Mitigation
Dec 11, 2020



Abstract:We propose a novel method for enforcing AI fairness with respect to protected or sensitive factors. This method uses a dual strategy performing Training And Representation Alteration (RENATA) for mitigation of two of the most prominent causes of AI bias, including: a) the use of representation learning alteration via adversarial independence, to suppress the bias-inducing dependence of the data representation from protected factors; and b) training set alteration via intelligent augmentation, to address bias-causing data imbalance, by using generative models that allow fine control of sensitive factors related to underrepresented populations. When testing our methods on image analytics, experiments demonstrate that RENATA significantly or fully debiases baseline models while outperforming competing debiasing methods, e.g., with (% overall accuracy, % accuracy gap) of (78.75, 0.5) vs. baseline method's (71.75, 10.5) for EyePACS, and (73.71, 11.82) vs. the (69.08, 21.65) baseline for CelebA. As an additional contribution, recognizing certain limitations in current metrics used for assessing debiasing performance, this study proposes novel conjunctive debiasing metrics. Our experiments also demonstrate the ability of these novel metrics in assessing the Pareto efficiency of the proposed methods.
Addressing Artificial Intelligence Bias in Retinal Disease Diagnostics
May 08, 2020



Abstract:This study evaluated novel AI and deep learning generative methods to address AI bias for retinal diagnostic applications when specifically applied to diabetic retinopathy (DR). Bias often results from data imbalance. We specifically considered here a strong form of data imbalance corresponding to domain shift, where AI classifiers are faced at inference time with data and concepts they were not trained on initially (here the concept of diseased black individuals). A baseline DR diagnostics DLS designed to solve a two-class problem of referable vs not referable DR was used. We modified the public domain Kaggle-EyePACS dataset (88,692 fundi and 44,346 individuals), which was originally designed to be diverse with regard to ethnicity, as follows: 1) we expanded it to include clinician-annotated labels for race since those were not publicly available; 2) we excluded training exemplars for diseased black individuals in training, but not testing, to construct a new scenario of data imbalance with domain shift. For this domain shifted scenario, the accuracy (95% confidence intervals [CI]) of the baseline DR diagnostics DLS for whites was 73.0% (66.9%,79.2%) vs. blacks of 60.5% (53.5%,67.3%], demonstrating disparity of AI performance as measured by accuracy across races. By contrast, an AI approach leveraging generative models was used to train a new diagnostic DLS with additional synthetically generated data for the missing subpopulation (diseased blacks), which achieved accuracy for whites of 77.5% (71.7%,83.3%) and for blacks of 70.0% (63.7%,76.4%), demonstrating closer parity in accuracy across races. The new debiased DLS also showed improvement in sensitivity of over 21% for blacks, with the same level of specificity, when compared with the baseline DLS. These findings demonstrate the potential benefits of using novel generative methods for debiasing AI.
Deep Learning based Retinal OCT Segmentation
Jan 29, 2018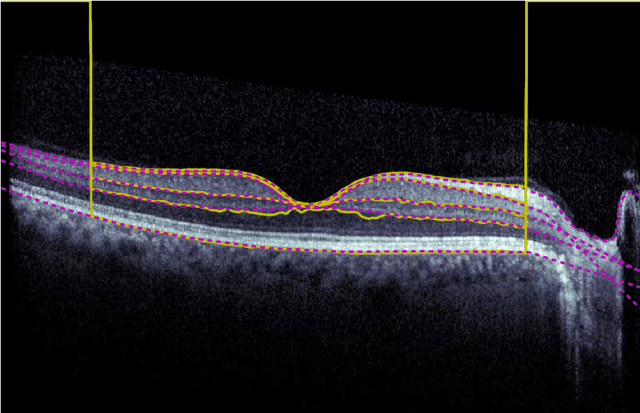
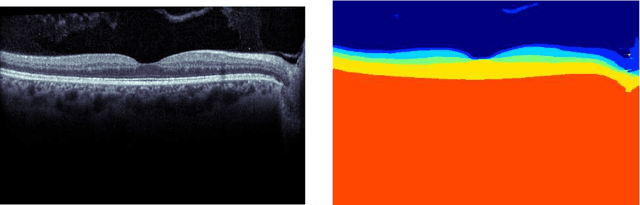
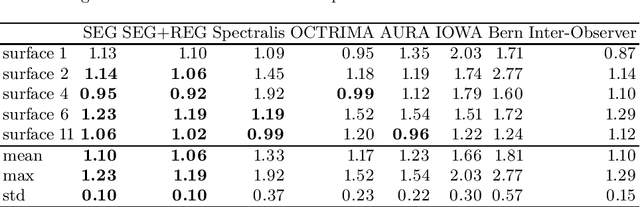
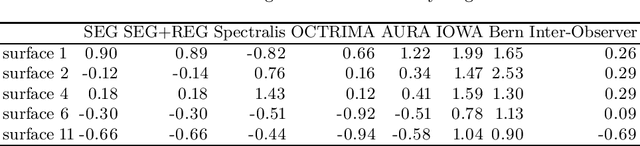
Abstract:Our objective is to evaluate the efficacy of methods that use deep learning (DL) for the automatic fine-grained segmentation of optical coherence tomography (OCT) images of the retina. OCT images from 10 patients with mild non-proliferative diabetic retinopathy were used from a public (U. of Miami) dataset. For each patient, five images were available: one image of the fovea center, two images of the perifovea, and two images of the parafovea. For each image, two expert graders each manually annotated five retinal surfaces (i.e. boundaries between pairs of retinal layers). The first grader's annotations were used as ground truth and the second grader's annotations to compute inter-operator agreement. The proposed automated approach segments images using fully convolutional networks (FCNs) together with Gaussian process (GP)-based regression as a post-processing step to improve the quality of the estimates. Using 10-fold cross validation, the performance of the algorithms is determined by computing the per-pixel unsigned error (distance) between the automated estimates and the ground truth annotations generated by the first manual grader. We compare the proposed method against five state of the art automatic segmentation techniques. The results show that the proposed methods compare favorably with state of the art techniques, resulting in the smallest mean unsigned error values and associated standard deviations, and performance is comparable with human annotation of retinal layers from OCT when there is only mild retinopathy. The results suggest that semantic segmentation using FCNs, coupled with regression-based post-processing, can effectively solve the OCT segmentation problem on par with human capabilities with mild retinopathy.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge