Delia Cabrera DeBuc
Block Expanded DINORET: Adapting Natural Domain Foundation Models for Retinal Imaging Without Catastrophic Forgetting
Sep 25, 2024
Abstract:Integrating deep learning into medical imaging is poised to greatly advance diagnostic methods but it faces challenges with generalizability. Foundation models, based on self-supervised learning, address these issues and improve data efficiency. Natural domain foundation models show promise for medical imaging, but systematic research evaluating domain adaptation, especially using self-supervised learning and parameter-efficient fine-tuning, remains underexplored. Additionally, little research addresses the issue of catastrophic forgetting during fine-tuning of foundation models. We adapted the DINOv2 vision transformer for retinal imaging classification tasks using self-supervised learning and generated two novel foundation models termed DINORET and BE DINORET. Publicly available color fundus photographs were employed for model development and subsequent fine-tuning for diabetic retinopathy staging and glaucoma detection. We introduced block expansion as a novel domain adaptation strategy and assessed the models for catastrophic forgetting. Models were benchmarked to RETFound, a state-of-the-art foundation model in ophthalmology. DINORET and BE DINORET demonstrated competitive performance on retinal imaging tasks, with the block expanded model achieving the highest scores on most datasets. Block expansion successfully mitigated catastrophic forgetting. Our few-shot learning studies indicated that DINORET and BE DINORET outperform RETFound in terms of data-efficiency. This study highlights the potential of adapting natural domain vision models to retinal imaging using self-supervised learning and block expansion. BE DINORET offers robust performance without sacrificing previously acquired capabilities. Our findings suggest that these methods could enable healthcare institutions to develop tailored vision models for their patient populations, enhancing global healthcare inclusivity.
Deep Learning based Retinal OCT Segmentation
Jan 29, 2018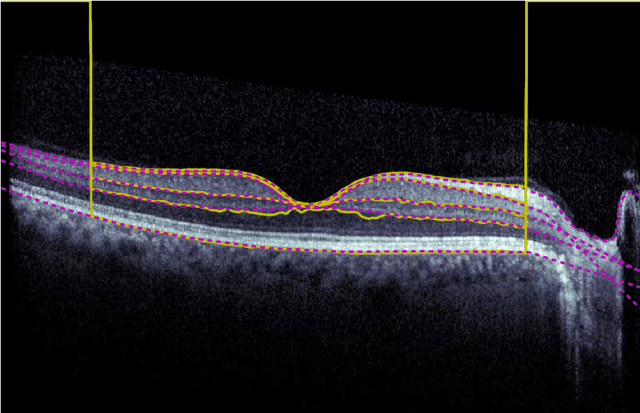
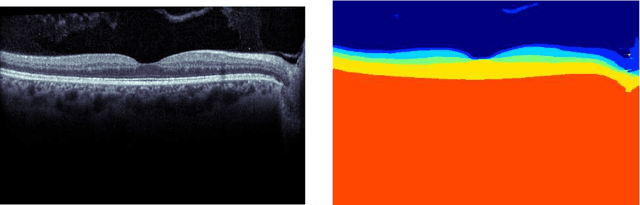
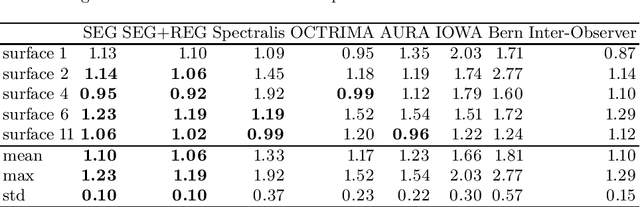
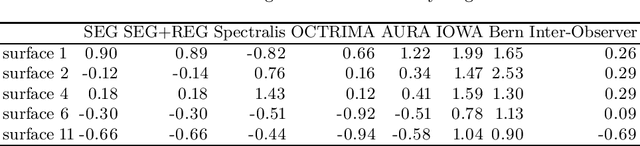
Abstract:Our objective is to evaluate the efficacy of methods that use deep learning (DL) for the automatic fine-grained segmentation of optical coherence tomography (OCT) images of the retina. OCT images from 10 patients with mild non-proliferative diabetic retinopathy were used from a public (U. of Miami) dataset. For each patient, five images were available: one image of the fovea center, two images of the perifovea, and two images of the parafovea. For each image, two expert graders each manually annotated five retinal surfaces (i.e. boundaries between pairs of retinal layers). The first grader's annotations were used as ground truth and the second grader's annotations to compute inter-operator agreement. The proposed automated approach segments images using fully convolutional networks (FCNs) together with Gaussian process (GP)-based regression as a post-processing step to improve the quality of the estimates. Using 10-fold cross validation, the performance of the algorithms is determined by computing the per-pixel unsigned error (distance) between the automated estimates and the ground truth annotations generated by the first manual grader. We compare the proposed method against five state of the art automatic segmentation techniques. The results show that the proposed methods compare favorably with state of the art techniques, resulting in the smallest mean unsigned error values and associated standard deviations, and performance is comparable with human annotation of retinal layers from OCT when there is only mild retinopathy. The results suggest that semantic segmentation using FCNs, coupled with regression-based post-processing, can effectively solve the OCT segmentation problem on par with human capabilities with mild retinopathy.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge