Naif Alkhunaizi
Probing the Efficacy of Federated Parameter-Efficient Fine-Tuning of Vision Transformers for Medical Image Classification
Jul 16, 2024



Abstract:With the advent of large pre-trained transformer models, fine-tuning these models for various downstream tasks is a critical problem. Paucity of training data, the existence of data silos, and stringent privacy constraints exacerbate this fine-tuning problem in the medical imaging domain, creating a strong need for algorithms that enable collaborative fine-tuning of pre-trained models. Moreover, the large size of these models necessitates the use of parameter-efficient fine-tuning (PEFT) to reduce the communication burden in federated learning. In this work, we systematically investigate various federated PEFT strategies for adapting a Vision Transformer (ViT) model (pre-trained on a large natural image dataset) for medical image classification. Apart from evaluating known PEFT techniques, we introduce new federated variants of PEFT algorithms such as visual prompt tuning (VPT), low-rank decomposition of visual prompts, stochastic block attention fine-tuning, and hybrid PEFT methods like low-rank adaptation (LoRA)+VPT. Moreover, we perform a thorough empirical analysis to identify the optimal PEFT method for the federated setting and understand the impact of data distribution on federated PEFT, especially for out-of-domain (OOD) and non-IID data. The key insight of this study is that while most federated PEFT methods work well for in-domain transfer, there is a substantial accuracy vs. efficiency trade-off when dealing with OOD and non-IID scenarios, which is commonly the case in medical imaging. Specifically, every order of magnitude reduction in fine-tuned/exchanged parameters can lead to a 4% drop in accuracy. Thus, the initial model choice is crucial for federated PEFT. It is preferable to use medical foundation models learned from in-domain medical image data (if available) rather than general vision models.
FedSIS: Federated Split Learning with Intermediate Representation Sampling for Privacy-preserving Generalized Face Presentation Attack Detection
Aug 22, 2023



Abstract:Lack of generalization to unseen domains/attacks is the Achilles heel of most face presentation attack detection (FacePAD) algorithms. Existing attempts to enhance the generalizability of FacePAD solutions assume that data from multiple source domains are available with a single entity to enable centralized training. In practice, data from different source domains may be collected by diverse entities, who are often unable to share their data due to legal and privacy constraints. While collaborative learning paradigms such as federated learning (FL) can overcome this problem, standard FL methods are ill-suited for domain generalization because they struggle to surmount the twin challenges of handling non-iid client data distributions during training and generalizing to unseen domains during inference. In this work, a novel framework called Federated Split learning with Intermediate representation Sampling (FedSIS) is introduced for privacy-preserving domain generalization. In FedSIS, a hybrid Vision Transformer (ViT) architecture is learned using a combination of FL and split learning to achieve robustness against statistical heterogeneity in the client data distributions without any sharing of raw data (thereby preserving privacy). To further improve generalization to unseen domains, a novel feature augmentation strategy called intermediate representation sampling is employed, and discriminative information from intermediate blocks of a ViT is distilled using a shared adapter network. The FedSIS approach has been evaluated on two well-known benchmarks for cross-domain FacePAD to demonstrate that it is possible to achieve state-of-the-art generalization performance without data sharing. Code: https://github.com/Naiftt/FedSIS
FeSViBS: Federated Split Learning of Vision Transformer with Block Sampling
Jun 26, 2023



Abstract:Data scarcity is a significant obstacle hindering the learning of powerful machine learning models in critical healthcare applications. Data-sharing mechanisms among multiple entities (e.g., hospitals) can accelerate model training and yield more accurate predictions. Recently, approaches such as Federated Learning (FL) and Split Learning (SL) have facilitated collaboration without the need to exchange private data. In this work, we propose a framework for medical imaging classification tasks called Federated Split learning of Vision transformer with Block Sampling (FeSViBS). The FeSViBS framework builds upon the existing federated split vision transformer and introduces a block sampling module, which leverages intermediate features extracted by the Vision Transformer (ViT) at the server. This is achieved by sampling features (patch tokens) from an intermediate transformer block and distilling their information content into a pseudo class token before passing them back to the client. These pseudo class tokens serve as an effective feature augmentation strategy and enhances the generalizability of the learned model. We demonstrate the utility of our proposed method compared to other SL and FL approaches on three publicly available medical imaging datasets: HAM1000, BloodMNIST, and Fed-ISIC2019, under both IID and non-IID settings. Code: https://github.com/faresmalik/FeSViBS
Suppressing Poisoning Attacks on Federated Learning for Medical Imaging
Jul 15, 2022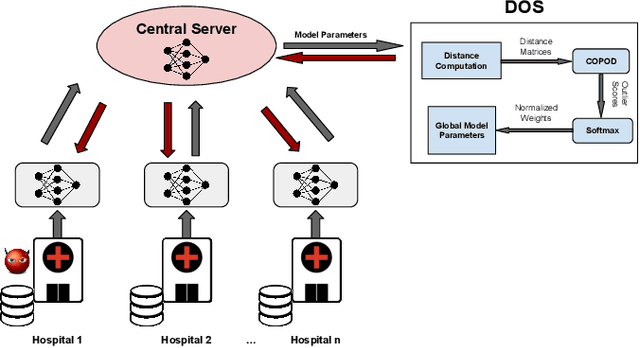
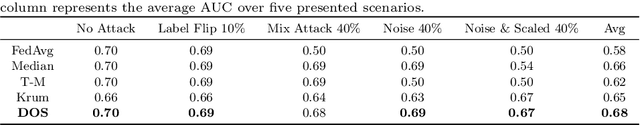
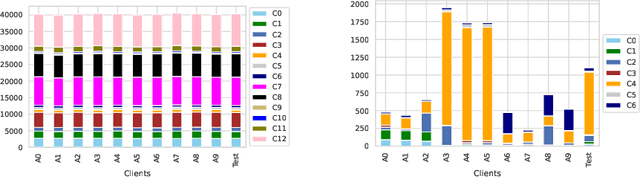
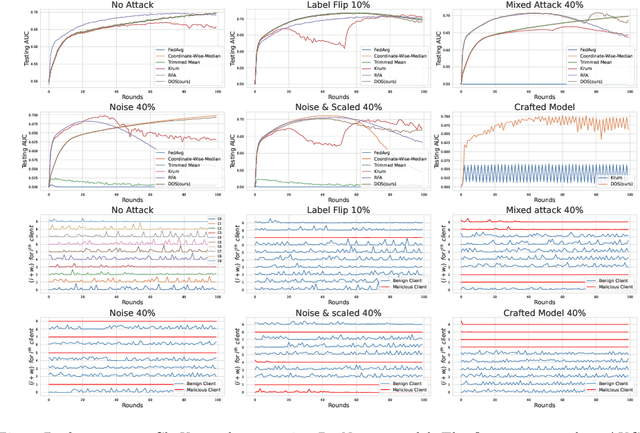
Abstract:Collaboration among multiple data-owning entities (e.g., hospitals) can accelerate the training process and yield better machine learning models due to the availability and diversity of data. However, privacy concerns make it challenging to exchange data while preserving confidentiality. Federated Learning (FL) is a promising solution that enables collaborative training through exchange of model parameters instead of raw data. However, most existing FL solutions work under the assumption that participating clients are \emph{honest} and thus can fail against poisoning attacks from malicious parties, whose goal is to deteriorate the global model performance. In this work, we propose a robust aggregation rule called Distance-based Outlier Suppression (DOS) that is resilient to byzantine failures. The proposed method computes the distance between local parameter updates of different clients and obtains an outlier score for each client using Copula-based Outlier Detection (COPOD). The resulting outlier scores are converted into normalized weights using a softmax function, and a weighted average of the local parameters is used for updating the global model. DOS aggregation can effectively suppress parameter updates from malicious clients without the need for any hyperparameter selection, even when the data distributions are heterogeneous. Evaluation on two medical imaging datasets (CheXpert and HAM10000) demonstrates the higher robustness of DOS method against a variety of poisoning attacks in comparison to other state-of-the-art methods. The code can be found here https://github.com/Naiftt/SPAFD.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge