Muhammad E. H. Chowdhury
Tracing 3D Anatomy in 2D Strokes: A Multi-Stage Projection Driven Approach to Cervical Spine Fracture Identification
Jan 21, 2026Abstract:Cervical spine fractures are critical medical conditions requiring precise and efficient detection for effective clinical management. This study explores the viability of 2D projection-based vertebra segmentation for vertebra-level fracture detection in 3D CT volumes, presenting an end-to-end pipeline for automated analysis of cervical vertebrae (C1-C7). By approximating a 3D volume through optimized 2D axial, sagittal, and coronal projections, regions of interest are identified using the YOLOv8 model from all views and combined to approximate the 3D cervical spine area, achieving a 3D mIoU of 94.45 percent. This projection-based localization strategy reduces computational complexity compared to traditional 3D segmentation methods while maintaining high performance. It is followed by a DenseNet121-Unet-based multi-label segmentation leveraging variance- and energy-based projections, achieving a Dice score of 87.86 percent. Strategic approximation of 3D vertebral masks from these 2D segmentation masks enables the extraction of individual vertebra volumes. The volumes are analyzed for fractures using an ensemble of 2.5D Spatio-Sequential models incorporating both raw slices and projections per vertebra for complementary evaluation. This ensemble achieves vertebra-level and patient-level F1 scores of 68.15 and 82.26, and ROC-AUC scores of 91.62 and 83.04, respectively. We further validate our approach through an explainability study that provides saliency map visualizations highlighting anatomical regions relevant for diagnosis, and an interobserver variability analysis comparing our model's performance with expert radiologists, demonstrating competitive results.
CASR-Net: An Image Processing-focused Deep Learning-based Coronary Artery Segmentation and Refinement Network for X-ray Coronary Angiogram
Oct 31, 2025Abstract:Early detection of coronary artery disease (CAD) is critical for reducing mortality and improving patient treatment planning. While angiographic image analysis from X-rays is a common and cost-effective method for identifying cardiac abnormalities, including stenotic coronary arteries, poor image quality can significantly impede clinical diagnosis. We present the Coronary Artery Segmentation and Refinement Network (CASR-Net), a three-stage pipeline comprising image preprocessing, segmentation, and refinement. A novel multichannel preprocessing strategy combining CLAHE and an improved Ben Graham method provides incremental gains, increasing Dice Score Coefficient (DSC) by 0.31-0.89% and Intersection over Union (IoU) by 0.40-1.16% compared with using the techniques individually. The core innovation is a segmentation network built on a UNet with a DenseNet121 encoder and a Self-organized Operational Neural Network (Self-ONN) based decoder, which preserves the continuity of narrow and stenotic vessel branches. A final contour refinement module further suppresses false positives. Evaluated with 5-fold cross-validation on a combination of two public datasets that contain both healthy and stenotic arteries, CASR-Net outperformed several state-of-the-art models, achieving an IoU of 61.43%, a DSC of 76.10%, and clDice of 79.36%. These results highlight a robust approach to automated coronary artery segmentation, offering a valuable tool to support clinicians in diagnosis and treatment planning.
GastroViT: A Vision Transformer Based Ensemble Learning Approach for Gastrointestinal Disease Classification with Grad CAM & SHAP Visualization
Sep 30, 2025Abstract:The gastrointestinal (GI) tract of humans can have a wide variety of aberrant mucosal abnormality findings, ranging from mild irritations to extremely fatal illnesses. Prompt identification of gastrointestinal disorders greatly contributes to arresting the progression of the illness and improving therapeutic outcomes. This paper presents an ensemble of pre-trained vision transformers (ViTs) for accurately classifying endoscopic images of the GI tract to categorize gastrointestinal problems and illnesses. ViTs, attention-based neural networks, have revolutionized image recognition by leveraging the transformative power of the transformer architecture, achieving state-of-the-art (SOTA) performance across various visual tasks. The proposed model was evaluated on the publicly available HyperKvasir dataset with 10,662 images of 23 different GI diseases for the purpose of identifying GI tract diseases. An ensemble method is proposed utilizing the predictions of two pre-trained models, MobileViT_XS and MobileViT_V2_200, which achieved accuracies of 90.57% and 90.48%, respectively. All the individual models are outperformed by the ensemble model, GastroViT, with an average precision, recall, F1 score, and accuracy of 69%, 63%, 64%, and 91.98%, respectively, in the first testing that involves 23 classes. The model comprises only 20 million (M) parameters, even without data augmentation and despite the highly imbalanced dataset. For the second testing with 16 classes, the scores are even higher, with average precision, recall, F1 score, and accuracy of 87%, 86%, 87%, and 92.70%, respectively. Additionally, the incorporation of explainable AI (XAI) methods such as Grad-CAM (Gradient Weighted Class Activation Mapping) and SHAP (Shapley Additive Explanations) enhances model interpretability, providing valuable insights for reliable GI diagnosis in real-world settings.
Deep Learning in Automated Power Line Inspection: A Review
Feb 10, 2025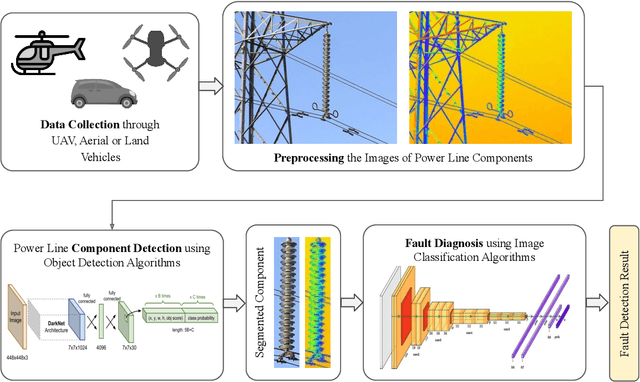



Abstract:In recent years, power line maintenance has seen a paradigm shift by moving towards computer vision-powered automated inspection. The utilization of an extensive collection of videos and images has become essential for maintaining the reliability, safety, and sustainability of electricity transmission. A significant focus on applying deep learning techniques for enhancing power line inspection processes has been observed in recent research. A comprehensive review of existing studies has been conducted in this paper, to aid researchers and industries in developing improved deep learning-based systems for analyzing power line data. The conventional steps of data analysis in power line inspections have been examined, and the body of current research has been systematically categorized into two main areas: the detection of components and the diagnosis of faults. A detailed summary of the diverse methods and techniques employed in these areas has been encapsulated, providing insights into their functionality and use cases. Special attention has been given to the exploration of deep learning-based methodologies for the analysis of power line inspection data, with an exposition of their fundamental principles and practical applications. Moreover, a vision for future research directions has been outlined, highlighting the need for advancements such as edge-cloud collaboration, and multi-modal analysis among others. Thus, this paper serves as a comprehensive resource for researchers delving into deep learning for power line analysis, illuminating the extent of current knowledge and the potential areas for future investigation.
Self-CephaloNet: A Two-stage Novel Framework using Operational Neural Network for Cephalometric Analysis
Jan 19, 2025Abstract:Cephalometric analysis is essential for the diagnosis and treatment planning of orthodontics. In lateral cephalograms, however, the manual detection of anatomical landmarks is a time-consuming procedure. Deep learning solutions hold the potential to address the time constraints associated with certain tasks; however, concerns regarding their performance have been observed. To address this critical issue, we proposed an end-to-end cascaded deep learning framework (Self-CepahloNet) for the task, which demonstrated benchmark performance over the ISBI 2015 dataset in predicting 19 dental landmarks. Due to their adaptive nodal capabilities, Self-ONN (self-operational neural networks) demonstrate superior learning performance for complex feature spaces over conventional convolutional neural networks. To leverage this attribute, we introduced a novel self-bottleneck in the HRNetV2 (High Resolution Network) backbone, which has exhibited benchmark performance on the ISBI 2015 dataset for the dental landmark detection task. Our first-stage results surpassed previous studies, showcasing the efficacy of our singular end-to-end deep learning model, which achieved a remarkable 70.95% success rate in detecting cephalometric landmarks within a 2mm range for the Test1 and Test2 datasets. Moreover, the second stage significantly improved overall performance, yielding an impressive 82.25% average success rate for the datasets above within the same 2mm distance. Furthermore, external validation was conducted using the PKU cephalogram dataset. Our model demonstrated a commendable success rate of 75.95% within the 2mm range.
Deep Learning-Driven Segmentation of Ischemic Stroke Lesions Using Multi-Channel MRI
Jan 04, 2025



Abstract:Ischemic stroke, caused by cerebral vessel occlusion, presents substantial challenges in medical imaging due to the variability and subtlety of stroke lesions. Magnetic Resonance Imaging (MRI) plays a crucial role in diagnosing and managing ischemic stroke, yet existing segmentation techniques often fail to accurately delineate lesions. This study introduces a novel deep learning-based method for segmenting ischemic stroke lesions using multi-channel MRI modalities, including Diffusion Weighted Imaging (DWI), Apparent Diffusion Coefficient (ADC), and enhanced Diffusion Weighted Imaging (eDWI). The proposed architecture integrates DenseNet121 as the encoder with Self-Organized Operational Neural Networks (SelfONN) in the decoder, enhanced by Channel and Space Compound Attention (CSCA) and Double Squeeze-and-Excitation (DSE) blocks. Additionally, a custom loss function combining Dice Loss and Jaccard Loss with weighted averages is introduced to improve model performance. Trained and evaluated on the ISLES 2022 dataset, the model achieved Dice Similarity Coefficients (DSC) of 83.88% using DWI alone, 85.86% with DWI and ADC, and 87.49% with the integration of DWI, ADC, and eDWI. This approach not only outperforms existing methods but also addresses key limitations in current segmentation practices. These advancements significantly enhance diagnostic precision and treatment planning for ischemic stroke, providing valuable support for clinical decision-making.
Ensemble Machine Learning Model for Inner Speech Recognition: A Subject-Specific Investigation
Dec 09, 2024



Abstract:Inner speech recognition has gained enormous interest in recent years due to its applications in rehabilitation, developing assistive technology, and cognitive assessment. However, since language and speech productions are a complex process, for which identifying speech components has remained a challenging task. Different approaches were taken previously to reach this goal, but new approaches remain to be explored. Also, a subject-oriented analysis is necessary to understand the underlying brain dynamics during inner speech production, which can bring novel methods to neurological research. A publicly available dataset, Thinking Out Loud Dataset, has been used to develop a Machine Learning (ML)-based technique to classify inner speech using 128-channel surface EEG signals. The dataset is collected on a Spanish cohort of ten subjects while uttering four words (Arriba, Abajo, Derecha, and Izquierda) by each participant. Statistical methods were employed to detect and remove motion artifacts from the Electroencephalography (EEG) signals. A large number (191 per channel) of time-, frequency- and time-frequency-domain features were extracted. Eight feature selection algorithms are explored, and the best feature selection technique is selected for subsequent evaluations. The performance of six ML algorithms is evaluated, and an ensemble model is proposed. Deep Learning (DL) models are also explored, and the results are compared with the classical ML approach. The proposed ensemble model, by stacking the five best logistic regression models, generated an overall accuracy of 81.13% and an F1 score of 81.12% in the classification of four inner speech words using surface EEG signals. The proposed framework with the proposed ensemble of classical ML models shows promise in the classification of inner speech using surface EEG signals.
Machine-agnostic Automated Lumbar MRI Segmentation using a Cascaded Model Based on Generative Neurons
Nov 23, 2024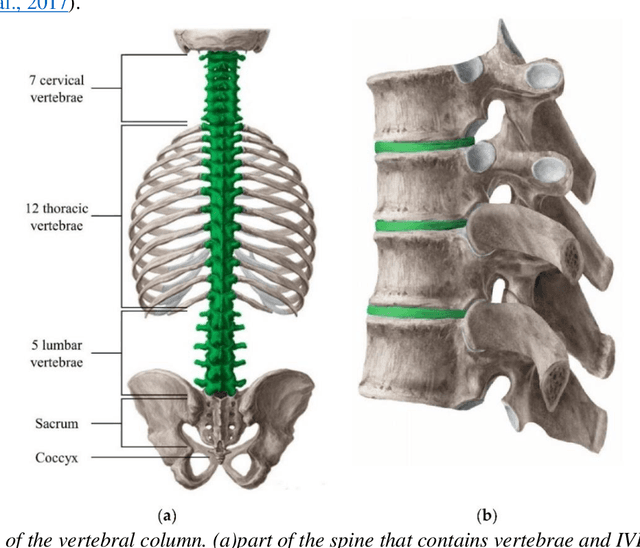
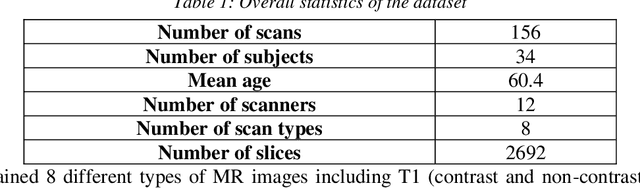
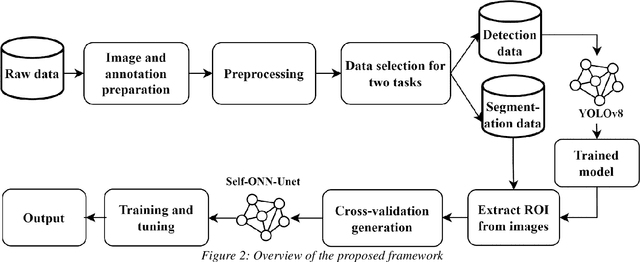
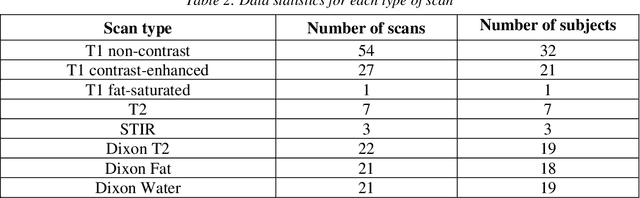
Abstract:Automated lumbar spine segmentation is very crucial for modern diagnosis systems. In this study, we introduce a novel machine-agnostic approach for segmenting lumbar vertebrae and intervertebral discs from MRI images, employing a cascaded model that synergizes an ROI detection and a Self-organized Operational Neural Network (Self-ONN)-based encoder-decoder network for segmentation. Addressing the challenge of diverse MRI modalities, our methodology capitalizes on a unique dataset comprising images from 12 scanners and 34 subjects, enhanced through strategic preprocessing and data augmentation techniques. The YOLOv8 medium model excels in ROI extraction, achieving an excellent performance of 0.916 mAP score. Significantly, our Self-ONN-based model, combined with a DenseNet121 encoder, demonstrates excellent performance in lumbar vertebrae and IVD segmentation with a mean Intersection over Union (IoU) of 83.66%, a sensitivity of 91.44%, and Dice Similarity Coefficient (DSC) of 91.03%, as validated through rigorous 10-fold cross-validation. This study not only showcases an effective approach to MRI segmentation in spine-related disorders but also sets the stage for future advancements in automated diagnostic tools, emphasizing the need for further dataset expansion and model refinement for broader clinical applicability.
Self-DenseMobileNet: A Robust Framework for Lung Nodule Classification using Self-ONN and Stacking-based Meta-Classifier
Oct 16, 2024Abstract:In this study, we propose a novel and robust framework, Self-DenseMobileNet, designed to enhance the classification of nodules and non-nodules in chest radiographs (CXRs). Our approach integrates advanced image standardization and enhancement techniques to optimize the input quality, thereby improving classification accuracy. To enhance predictive accuracy and leverage the strengths of multiple models, the prediction probabilities from Self-DenseMobileNet were transformed into tabular data and used to train eight classical machine learning (ML) models; the top three performers were then combined via a stacking algorithm, creating a robust meta-classifier that integrates their collective insights for superior classification performance. To enhance the interpretability of our results, we employed class activation mapping (CAM) to visualize the decision-making process of the best-performing model. Our proposed framework demonstrated remarkable performance on internal validation data, achieving an accuracy of 99.28\% using a Meta-Random Forest Classifier. When tested on an external dataset, the framework maintained strong generalizability with an accuracy of 89.40\%. These results highlight a significant improvement in the classification of CXRs with lung nodules.
Advanced Artificial Intelligence Algorithms in Cochlear Implants: Review of Healthcare Strategies, Challenges, and Perspectives
Mar 17, 2024



Abstract:Automatic speech recognition (ASR) plays a pivotal role in our daily lives, offering utility not only for interacting with machines but also for facilitating communication for individuals with either partial or profound hearing impairments. The process involves receiving the speech signal in analogue form, followed by various signal processing algorithms to make it compatible with devices of limited capacity, such as cochlear implants (CIs). Unfortunately, these implants, equipped with a finite number of electrodes, often result in speech distortion during synthesis. Despite efforts by researchers to enhance received speech quality using various state-of-the-art signal processing techniques, challenges persist, especially in scenarios involving multiple sources of speech, environmental noise, and other circumstances. The advent of new artificial intelligence (AI) methods has ushered in cutting-edge strategies to address the limitations and difficulties associated with traditional signal processing techniques dedicated to CIs. This review aims to comprehensively review advancements in CI-based ASR and speech enhancement, among other related aspects. The primary objective is to provide a thorough overview of metrics and datasets, exploring the capabilities of AI algorithms in this biomedical field, summarizing and commenting on the best results obtained. Additionally, the review will delve into potential applications and suggest future directions to bridge existing research gaps in this domain.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge