Miika T. Nieminen
End-To-End Prediction of Knee Osteoarthritis Progression With Multi-Modal Transformers
Jul 03, 2023Abstract:Knee Osteoarthritis (KOA) is a highly prevalent chronic musculoskeletal condition with no currently available treatment. The manifestation of KOA is heterogeneous and prediction of its progression is challenging. Current literature suggests that the use of multi-modal data and advanced modeling methods, such as the ones based on Deep Learning, has promise in tackling this challenge. To date, however, the evidence on the efficacy of this approach is limited. In this study, we leveraged recent advances in Deep Learning and, using a Transformer approach, developed a unified framework for the multi-modal fusion of knee imaging data. Subsequently, we analyzed its performance across a range of scenarios by investigating multiple progression horizons -- from short-term to long-term. We report our findings using a large cohort (n=2421-3967) derived from the Osteoarthritis Initiative dataset. We show that structural knee MRI allows identifying radiographic KOA progressors on par with multi-modal fusion approaches, achieving an area under the ROC curve (ROC AUC) of 0.70-0.76 and Average Precision (AP) of 0.15-0.54 in 2-8 year horizons. Progression within 1 year was better predicted with a multi-modal method using X-ray, structural, and compositional MR images -- ROC AUC of 0.76(0.04), AP of 0.13(0.04) -- or via clinical data. Our follow-up analysis generally shows that prediction from the imaging data is more accurate for post-traumatic subjects, and we further investigate which subject subgroups may benefit the most. The present study provides novel insights into multi-modal imaging of KOA and brings a unified data-driven framework for studying its progression in an end-to-end manner, providing new tools for the design of more efficient clinical trials. The source code of our framework and the pre-trained models are made publicly available.
Unsupervised denoising for sparse multi-spectral computed tomography
Nov 02, 2022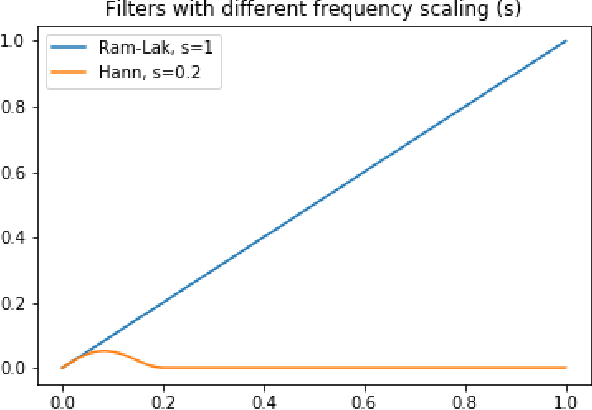
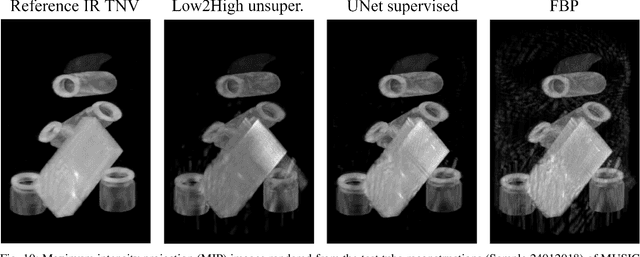
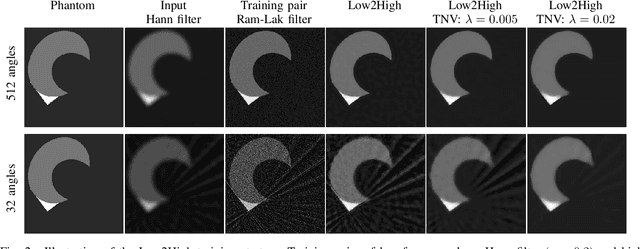
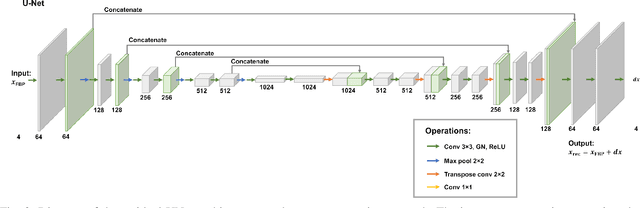
Abstract:Multi-energy computed tomography (CT) with photon counting detectors (PCDs) enables spectral imaging as PCDs can assign the incoming photons to specific energy channels. However, PCDs with many spectral channels drastically increase the computational complexity of the CT reconstruction, and bespoke reconstruction algorithms need fine-tuning to varying noise statistics. \rev{Especially if many projections are taken, a large amount of data has to be collected and stored. Sparse view CT is one solution for data reduction. However, these issues are especially exacerbated when sparse imaging scenarios are encountered due to a significant reduction in photon counts.} In this work, we investigate the suitability of learning-based improvements to the challenging task of obtaining high-quality reconstructions from sparse measurements for a 64-channel PCD-CT. In particular, to overcome missing reference data for the training procedure, we propose an unsupervised denoising and artefact removal approach by exploiting different filter functions in the reconstruction and an explicit coupling of spectral channels with the nuclear norm. Performance is assessed on both simulated synthetic data and the openly available experimental Multi-Spectral Imaging via Computed Tomography (MUSIC) dataset. We compared the quality of our unsupervised method to iterative total nuclear variation regularized reconstructions and a supervised denoiser trained with reference data. We show that improved reconstruction quality can be achieved with flexibility on noise statistics and effective suppression of streaking artefacts when using unsupervised denoising with spectral coupling.
Predicting Knee Osteoarthritis Progression from Structural MRI using Deep Learning
Jan 26, 2022



Abstract:Accurate prediction of knee osteoarthritis (KOA) progression from structural MRI has a potential to enhance disease understanding and support clinical trials. Prior art focused on manually designed imaging biomarkers, which may not fully exploit all disease-related information present in MRI scan. In contrast, our method learns relevant representations from raw data end-to-end using Deep Learning, and uses them for progression prediction. The method employs a 2D CNN to process the data slice-wise and aggregate the extracted features using a Transformer. Evaluated on a large cohort (n=4,866), the proposed method outperforms conventional 2D and 3D CNN-based models and achieves average precision of $0.58\pm0.03$ and ROC AUC of $0.78\pm0.01$. This paper sets a baseline on end-to-end KOA progression prediction from structural MRI. Our code is publicly available at https://github.com/MIPT-Oulu/OAProgressionMR.
Machine Learning Based Texture Analysis of Patella from X-Rays for Detecting Patellofemoral Osteoarthritis
Jun 04, 2021



Abstract:Objective is to assess the ability of texture features for detecting radiographic patellofemoral osteoarthritis (PFOA) from knee lateral view radiographs. We used lateral view knee radiographs from MOST public use datasets (n = 5507 knees). Patellar region-of-interest (ROI) was automatically detected using landmark detection tool (BoneFinder). Hand-crafted features, based on LocalBinary Patterns (LBP), were then extracted to describe the patellar texture. First, a machine learning model (Gradient Boosting Machine) was trained to detect radiographic PFOA from the LBP features. Furthermore, we used end-to-end trained deep convolutional neural networks (CNNs) directly on the texture patches for detecting the PFOA. The proposed classification models were eventually compared with more conventional reference models that use clinical assessments and participant characteristics such as age, sex, body mass index(BMI), the total WOMAC score, and tibiofemoral Kellgren-Lawrence (KL) grade. Atlas-guided visual assessment of PFOA status by expert readers provided in the MOST public use datasets was used as a classification outcome for the models. Performance of prediction models was assessed using the area under the receiver operating characteristic curve (ROC AUC), the area under the precision-recall (PR) curve-average precision (AP)-, and Brier score in the stratified 5-fold cross validation setting.Of the 5507 knees, 953 (17.3%) had PFOA. AUC and AP for the strongest reference model including age, sex, BMI, WOMAC score, and tibiofemoral KL grade to predict PFOA were 0.817 and 0.487, respectively. Textural ROI classification using CNN significantly improved the prediction performance (ROC AUC= 0.889, AP= 0.714). We present the first study that analyses patellar bone texture for diagnosing PFOA. Our results demonstrates the potential of using texture features of patella to predict PFOA.
Automated Detection of Patellofemoral Osteoarthritis from Knee Lateral View Radiographs Using Deep Learning: Data from the Multicenter Osteoarthritis Study (MOST)
Jan 12, 2021



Abstract:Objective: To assess the ability of imaging-based deep learning to predict radiographic patellofemoral osteoarthritis (PFOA) from knee lateral view radiographs. Design: Knee lateral view radiographs were extracted from The Multicenter Osteoarthritis Study (MOST) (n = 18,436 knees). Patellar region-of-interest (ROI) was first automatically detected, and subsequently, end-to-end deep convolutional neural networks (CNNs) were trained and validated to detect the status of patellofemoral OA. Patellar ROI was detected using deep-learning-based object detection method. Manual PFOA status assessment provided in the MOST dataset was used as a classification outcome for the CNNs. Performance of prediction models was assessed using the area under the receiver operating characteristic curve (ROC AUC) and the average precision (AP) obtained from the precision-recall (PR) curve in the stratified 5-fold cross validation setting. Results: Of the 18,436 knees, 3,425 (19%) had PFOA. AUC and AP for the reference model including age, sex, body mass index (BMI), the total Western Ontario and McMaster Universities Arthritis Index (WOMAC) score, and tibiofemoral Kellgren-Lawrence (KL) grade to predict PFOA were 0.806 and 0.478, respectively. The CNN model that used only image data significantly improved the prediction of PFOA status (ROC AUC= 0.958, AP= 0.862). Conclusion: We present the first machine learning based automatic PFOA detection method. Furthermore, our deep learning based model trained on patella region from knee lateral view radiographs performs better at predicting PFOA than models based on patient characteristics and clinical assessments.
A Lightweight CNN and Joint Shape-Joint Space Descriptor for Radiological Osteoarthritis Detection
May 24, 2020



Abstract:Knee osteoarthritis (OA) is very common progressive and degenerative musculoskeletal disease worldwide creates a heavy burden on patients with reduced quality of life and also on society due to financial impact. Therefore, any attempt to reduce the burden of the disease could help both patients and society. In this study, we propose a fully automated novel method, based on combination of joint shape and convolutional neural network (CNN) based bone texture features, to distinguish between the knee radiographs with and without radiographic osteoarthritis. Moreover, we report the first attempt at describing the bone texture using CNN. Knee radiographs from Osteoarthritis Initiative (OAI) and Multicenter Osteoarthritis (MOST) studies were used in the experiments. Our models were trained on 8953 knee radiographs from OAI and evaluated on 3445 knee radiographs from MOST. Our results demonstrate that fusing the proposed shape and texture parameters achieves the state-of-the art performance in radiographic OA detection yielding area under the ROC curve (AUC) of 95.21%
Adaptive Segmentation of Knee Radiographs for Selecting the Optimal ROI in Texture Analysis
Aug 21, 2019



Abstract:The purposes of this study were to investigate: 1) the effect of placement of region-of-interest (ROI) for texture analysis of subchondral bone in knee radiographs, and 2) the ability of several texture descriptors to distinguish between the knees with and without radiographic osteoarthritis (OA). Bilateral posterior-anterior knee radiographs were analyzed from the baseline of OAI and MOST datasets. A fully automatic method to locate the most informative region from subchondral bone using adaptive segmentation was developed. We used an oversegmentation strategy for partitioning knee images into the compact regions that follow natural texture boundaries. LBP, Fractal Dimension (FD), Haralick features, Shannon entropy, and HOG methods were computed within the standard ROI and within the proposed adaptive ROIs. Subsequently, we built logistic regression models to identify and compare the performances of each texture descriptor and each ROI placement method using 5-fold cross validation setting. Importantly, we also investigated the generalizability of our approach by training the models on OAI and testing them on MOST dataset.We used area under the receiver operating characteristic (ROC) curve (AUC) and average precision (AP) obtained from the precision-recall (PR) curve to compare the results. We found that the adaptive ROI improves the classification performance (OA vs. non-OA) over the commonly used standard ROI (up to 9% percent increase in AUC). We also observed that, from all texture parameters, LBP yielded the best performance in all settings with the best AUC of 0.840 [0.825, 0.852] and associated AP of 0.804 [0.786, 0.820]. Compared to the current state-of-the-art approaches, our results suggest that the proposed adaptive ROI approach in texture analysis of subchondral bone can increase the diagnostic performance for detecting the presence of radiographic OA.
Improving Robustness of Deep Learning Based Knee MRI Segmentation: Mixup and Adversarial Domain Adaptation
Aug 14, 2019
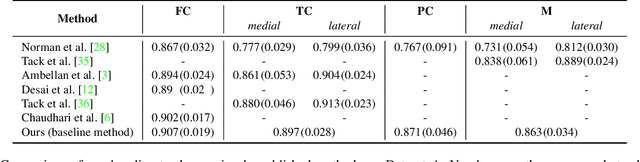
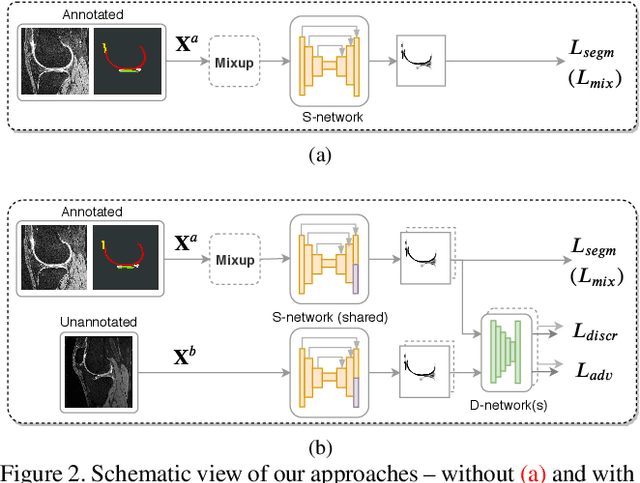
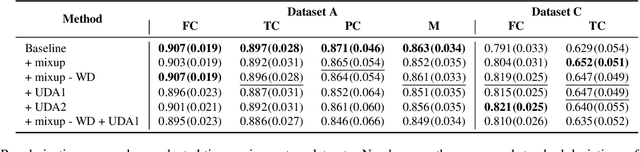
Abstract:Degeneration of articular cartilage (AC) is actively studied in knee osteoarthritis (OA) research via magnetic resonance imaging (MRI). Segmentation of AC tissues from MRI data is an essential step in quantification of their damage. Deep learning (DL) based methods have shown potential in this realm and are the current state-of-the-art, however, their robustness to heterogeneity of MRI acquisition settings remains an open problem. In this study, we investigated two modern regularization techniques -- mixup and adversarial unsupervised domain adaptation (UDA) -- to improve the robustness of DL-based knee cartilage segmentation to new MRI acquisition settings. Our validation setup included two datasets produced by different MRI scanners and using distinct data acquisition protocols. We assessed the robustness of automatic segmentation by comparing mixup and UDA approaches to a strong baseline method at different OA severity stages and, additionally, in relation to anatomical locations. Our results showed that for moderate changes in knee MRI data acquisition settings both approaches may provide notable improvements in the robustness, which are consistent for all stages of the disease and affect the clinically important areas of the knee joint. However, mixup may be considered as a recommended approach, since it is more computationally efficient and does not require additional data from the target acquisition setup.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge