Simo Saarakkala
End-To-End Prediction of Knee Osteoarthritis Progression With Multi-Modal Transformers
Jul 03, 2023



Abstract:Knee Osteoarthritis (KOA) is a highly prevalent chronic musculoskeletal condition with no currently available treatment. The manifestation of KOA is heterogeneous and prediction of its progression is challenging. Current literature suggests that the use of multi-modal data and advanced modeling methods, such as the ones based on Deep Learning, has promise in tackling this challenge. To date, however, the evidence on the efficacy of this approach is limited. In this study, we leveraged recent advances in Deep Learning and, using a Transformer approach, developed a unified framework for the multi-modal fusion of knee imaging data. Subsequently, we analyzed its performance across a range of scenarios by investigating multiple progression horizons -- from short-term to long-term. We report our findings using a large cohort (n=2421-3967) derived from the Osteoarthritis Initiative dataset. We show that structural knee MRI allows identifying radiographic KOA progressors on par with multi-modal fusion approaches, achieving an area under the ROC curve (ROC AUC) of 0.70-0.76 and Average Precision (AP) of 0.15-0.54 in 2-8 year horizons. Progression within 1 year was better predicted with a multi-modal method using X-ray, structural, and compositional MR images -- ROC AUC of 0.76(0.04), AP of 0.13(0.04) -- or via clinical data. Our follow-up analysis generally shows that prediction from the imaging data is more accurate for post-traumatic subjects, and we further investigate which subject subgroups may benefit the most. The present study provides novel insights into multi-modal imaging of KOA and brings a unified data-driven framework for studying its progression in an end-to-end manner, providing new tools for the design of more efficient clinical trials. The source code of our framework and the pre-trained models are made publicly available.
Deep Learning for Predicting Progression of Patellofemoral Osteoarthritis Based on Lateral Knee Radiographs, Demographic Data and Symptomatic Assessments
May 10, 2023Abstract:In this study, we propose a novel framework that utilizes deep learning (DL) and attention mechanisms to predict the radiographic progression of patellofemoral osteoarthritis (PFOA) over a period of seven years. This study included subjects (1832 subjects, 3276 knees) from the baseline of the MOST study. PF joint regions-of-interest were identified using an automated landmark detection tool (BoneFinder) on lateral knee X-rays. An end-to-end DL method was developed for predicting PFOA progression based on imaging data in a 5-fold cross-validation setting. A set of baselines based on known risk factors were developed and analyzed using gradient boosting machine (GBM). Risk factors included age, sex, BMI and WOMAC score, and the radiographic osteoarthritis stage of the tibiofemoral joint (KL score). Finally, we trained an ensemble model using both imaging and clinical data. Among the individual models, the performance of our deep convolutional neural network attention model achieved the best performance with an AUC of 0.856 and AP of 0.431; slightly outperforming the deep learning approach without attention (AUC=0.832, AP= 0.4) and the best performing reference GBM model (AUC=0.767, AP= 0.334). The inclusion of imaging data and clinical variables in an ensemble model allowed statistically more powerful prediction of PFOA progression (AUC = 0.865, AP=0.447), although the clinical significance of this minor performance gain remains unknown. This study demonstrated the potential of machine learning models to predict the progression of PFOA using imaging and clinical variables. These models could be used to identify patients who are at high risk of progression and prioritize them for new treatments. However, even though the accuracy of the models were excellent in this study using the MOST dataset, they should be still validated using external patient cohorts in the future.
A Stronger Baseline For Automatic Pfirrmann Grading Of Lumbar Spine MRI Using Deep Learning
Oct 26, 2022
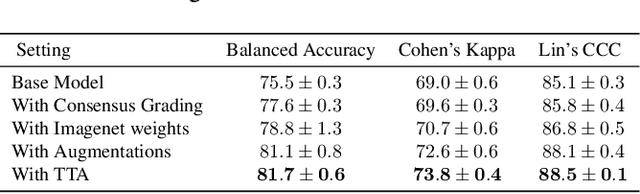
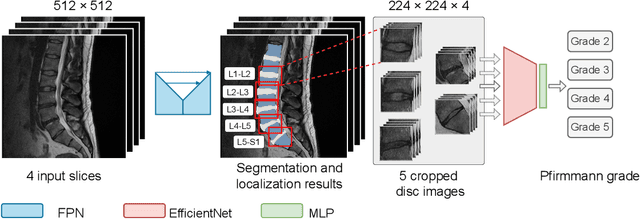
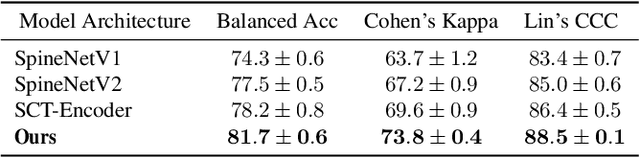
Abstract:This paper addresses the challenge of grading visual features in lumbar spine MRI using Deep Learning. Such a method is essential for the automatic quantification of structural changes in the spine, which is valuable for understanding low back pain. Multiple recent studies investigated different architecture designs, and the most recent success has been attributed to the use of transformer architectures. In this work, we argue that with a well-tuned three-stage pipeline comprising semantic segmentation, localization, and classification, convolutional networks outperform the state-of-the-art approaches. We conducted an ablation study of the existing methods in a population cohort, and report performance generalization across various subgroups. Our code is publicly available to advance research on disc degeneration and low back pain.
Clinically-Inspired Multi-Agent Transformers for Disease Trajectory Forecasting from Multimodal Data
Oct 25, 2022Abstract:Deep neural networks are often applied to medical images to automate the problem of medical diagnosis. However, a more clinically relevant question that practitioners usually face is how to predict the future trajectory of a disease. Current methods for prognosis or disease trajectory forecasting often require domain knowledge and are complicated to apply. In this paper, we formulate the prognosis prediction problem as a one-to-many prediction problem. Inspired by a clinical decision-making process with two agents -- a radiologist and a general practitioner -- we predict prognosis with two transformer-based components that share information with each other. The first transformer in this framework aims to analyze the imaging data, and the second one leverages its internal states as inputs, also fusing them with auxiliary clinical data. The temporal nature of the problem is modeled within the transformer states, allowing us to treat the forecasting problem as a multi-task classification, for which we propose a novel loss. We show the effectiveness of our approach in predicting the development of structural knee osteoarthritis changes and forecasting Alzheimer's disease clinical status directly from raw multi-modal data. The proposed method outperforms multiple state-of-the-art baselines with respect to performance and calibration, both of which are needed for real-world applications. An open-source implementation of our method is made publicly available at \url{https://github.com/Oulu-IMEDS/CLIMATv2}.
Predicting Knee Osteoarthritis Progression from Structural MRI using Deep Learning
Jan 26, 2022



Abstract:Accurate prediction of knee osteoarthritis (KOA) progression from structural MRI has a potential to enhance disease understanding and support clinical trials. Prior art focused on manually designed imaging biomarkers, which may not fully exploit all disease-related information present in MRI scan. In contrast, our method learns relevant representations from raw data end-to-end using Deep Learning, and uses them for progression prediction. The method employs a 2D CNN to process the data slice-wise and aggregate the extracted features using a Transformer. Evaluated on a large cohort (n=4,866), the proposed method outperforms conventional 2D and 3D CNN-based models and achieves average precision of $0.58\pm0.03$ and ROC AUC of $0.78\pm0.01$. This paper sets a baseline on end-to-end KOA progression prediction from structural MRI. Our code is publicly available at https://github.com/MIPT-Oulu/OAProgressionMR.
Machine Learning Based Texture Analysis of Patella from X-Rays for Detecting Patellofemoral Osteoarthritis
Jun 04, 2021



Abstract:Objective is to assess the ability of texture features for detecting radiographic patellofemoral osteoarthritis (PFOA) from knee lateral view radiographs. We used lateral view knee radiographs from MOST public use datasets (n = 5507 knees). Patellar region-of-interest (ROI) was automatically detected using landmark detection tool (BoneFinder). Hand-crafted features, based on LocalBinary Patterns (LBP), were then extracted to describe the patellar texture. First, a machine learning model (Gradient Boosting Machine) was trained to detect radiographic PFOA from the LBP features. Furthermore, we used end-to-end trained deep convolutional neural networks (CNNs) directly on the texture patches for detecting the PFOA. The proposed classification models were eventually compared with more conventional reference models that use clinical assessments and participant characteristics such as age, sex, body mass index(BMI), the total WOMAC score, and tibiofemoral Kellgren-Lawrence (KL) grade. Atlas-guided visual assessment of PFOA status by expert readers provided in the MOST public use datasets was used as a classification outcome for the models. Performance of prediction models was assessed using the area under the receiver operating characteristic curve (ROC AUC), the area under the precision-recall (PR) curve-average precision (AP)-, and Brier score in the stratified 5-fold cross validation setting.Of the 5507 knees, 953 (17.3%) had PFOA. AUC and AP for the strongest reference model including age, sex, BMI, WOMAC score, and tibiofemoral KL grade to predict PFOA were 0.817 and 0.487, respectively. Textural ROI classification using CNN significantly improved the prediction performance (ROC AUC= 0.889, AP= 0.714). We present the first study that analyses patellar bone texture for diagnosing PFOA. Our results demonstrates the potential of using texture features of patella to predict PFOA.
DeepProg: A Transformer-based Framework for Predicting Disease Prognosis
Apr 08, 2021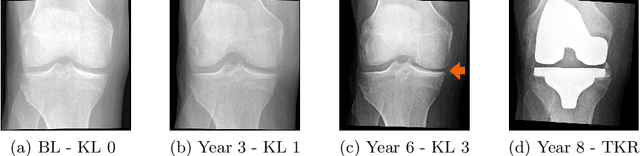

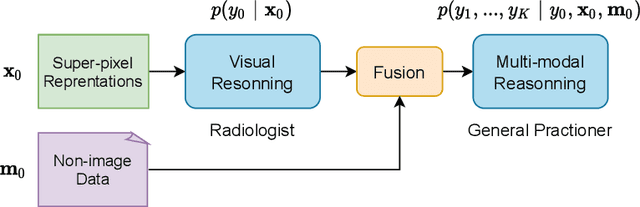
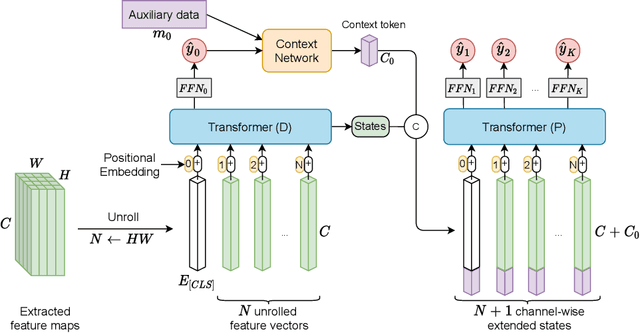
Abstract:A vast majority of deep learning methods are built to automate diagnostic tasks. However, in clinical practice, a more advanced question is how to predict the course of a disease. Current methods for this problem are complicated, and often require domain knowledge, making them difficult for practitioners to use. In this paper, we formulate the prognosis prediction task as a one-to-many sequence prediction problem. Inspired by a clinical decision making process with two agents -- a radiologist and a general practitioner -- we propose a generic end-to-end transformer-based framework to estimate disease prognosis from images and auxiliary data. The effectiveness and validation of the developed method are shown on synthetic data, and in the task of predicting the development of structural osteoarthritic changes in knee joints.
Automated Detection of Patellofemoral Osteoarthritis from Knee Lateral View Radiographs Using Deep Learning: Data from the Multicenter Osteoarthritis Study (MOST)
Jan 12, 2021



Abstract:Objective: To assess the ability of imaging-based deep learning to predict radiographic patellofemoral osteoarthritis (PFOA) from knee lateral view radiographs. Design: Knee lateral view radiographs were extracted from The Multicenter Osteoarthritis Study (MOST) (n = 18,436 knees). Patellar region-of-interest (ROI) was first automatically detected, and subsequently, end-to-end deep convolutional neural networks (CNNs) were trained and validated to detect the status of patellofemoral OA. Patellar ROI was detected using deep-learning-based object detection method. Manual PFOA status assessment provided in the MOST dataset was used as a classification outcome for the CNNs. Performance of prediction models was assessed using the area under the receiver operating characteristic curve (ROC AUC) and the average precision (AP) obtained from the precision-recall (PR) curve in the stratified 5-fold cross validation setting. Results: Of the 18,436 knees, 3,425 (19%) had PFOA. AUC and AP for the reference model including age, sex, body mass index (BMI), the total Western Ontario and McMaster Universities Arthritis Index (WOMAC) score, and tibiofemoral Kellgren-Lawrence (KL) grade to predict PFOA were 0.806 and 0.478, respectively. The CNN model that used only image data significantly improved the prediction of PFOA status (ROC AUC= 0.958, AP= 0.862). Conclusion: We present the first machine learning based automatic PFOA detection method. Furthermore, our deep learning based model trained on patella region from knee lateral view radiographs performs better at predicting PFOA than models based on patient characteristics and clinical assessments.
A Lightweight CNN and Joint Shape-Joint Space Descriptor for Radiological Osteoarthritis Detection
May 24, 2020



Abstract:Knee osteoarthritis (OA) is very common progressive and degenerative musculoskeletal disease worldwide creates a heavy burden on patients with reduced quality of life and also on society due to financial impact. Therefore, any attempt to reduce the burden of the disease could help both patients and society. In this study, we propose a fully automated novel method, based on combination of joint shape and convolutional neural network (CNN) based bone texture features, to distinguish between the knee radiographs with and without radiographic osteoarthritis. Moreover, we report the first attempt at describing the bone texture using CNN. Knee radiographs from Osteoarthritis Initiative (OAI) and Multicenter Osteoarthritis (MOST) studies were used in the experiments. Our models were trained on 8953 knee radiographs from OAI and evaluated on 3445 knee radiographs from MOST. Our results demonstrate that fusing the proposed shape and texture parameters achieves the state-of-the art performance in radiographic OA detection yielding area under the ROC curve (AUC) of 95.21%
Semixup: In- and Out-of-Manifold Regularization for Deep Semi-Supervised Knee Osteoarthritis Severity Grading from Plain Radiographs
Mar 05, 2020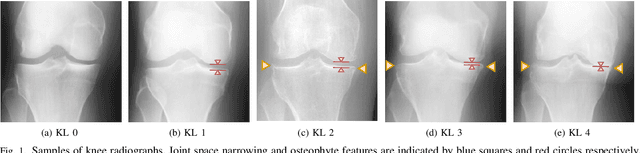
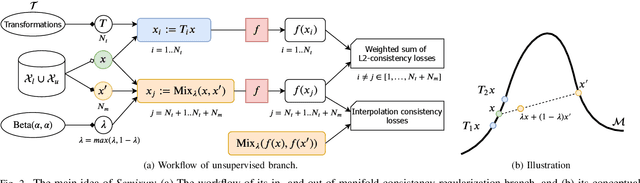
Abstract:Knee osteoarthritis (OA) is one of the highest disability factors in the world in humans. This musculoskeletal disorder is assessed from clinical symptoms, and typically confirmed via radiographic assessment. This visual assessment done by a radiologist requires experience, and suffers from high inter-observer variability. The recent development in the literature has shown that deep learning (DL) methods can reliably perform the OA severity assessment according to the gold standard Kellgren-Lawrence (KL) grading system. However, these methods require large amounts of labeled data, which are costly to obtain. In this study, we propose the Semixup algorithm, a semi-supervised learning (SSL) approach to leverage unlabeled data. Semixup relies on consistency regularization using in- and out-of-manifold samples, together with interpolated consistency. On an independent test set, our method significantly outperformed other state-of-the-art SSL methods in most cases, and even achieved a comparable performance to a well-tuned fully supervised learning (SL) model that required over 12 times more labeled data.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge