Michael Iv
Automated Real-time Assessment of Intracranial Hemorrhage Detection AI Using an Ensembled Monitoring Model (EMM)
May 16, 2025Abstract:Artificial intelligence (AI) tools for radiology are commonly unmonitored once deployed. The lack of real-time case-by-case assessments of AI prediction confidence requires users to independently distinguish between trustworthy and unreliable AI predictions, which increases cognitive burden, reduces productivity, and potentially leads to misdiagnoses. To address these challenges, we introduce Ensembled Monitoring Model (EMM), a framework inspired by clinical consensus practices using multiple expert reviews. Designed specifically for black-box commercial AI products, EMM operates independently without requiring access to internal AI components or intermediate outputs, while still providing robust confidence measurements. Using intracranial hemorrhage detection as our test case on a large, diverse dataset of 2919 studies, we demonstrate that EMM successfully categorizes confidence in the AI-generated prediction, suggesting different actions and helping improve the overall performance of AI tools to ultimately reduce cognitive burden. Importantly, we provide key technical considerations and best practices for successfully translating EMM into clinical settings.
Analysis of the MICCAI Brain Tumor Segmentation -- Metastases (BraTS-METS) 2025 Lighthouse Challenge: Brain Metastasis Segmentation on Pre- and Post-treatment MRI
Apr 16, 2025Abstract:Despite continuous advancements in cancer treatment, brain metastatic disease remains a significant complication of primary cancer and is associated with an unfavorable prognosis. One approach for improving diagnosis, management, and outcomes is to implement algorithms based on artificial intelligence for the automated segmentation of both pre- and post-treatment MRI brain images. Such algorithms rely on volumetric criteria for lesion identification and treatment response assessment, which are still not available in clinical practice. Therefore, it is critical to establish tools for rapid volumetric segmentations methods that can be translated to clinical practice and that are trained on high quality annotated data. The BraTS-METS 2025 Lighthouse Challenge aims to address this critical need by establishing inter-rater and intra-rater variability in dataset annotation by generating high quality annotated datasets from four individual instances of segmentation by neuroradiologists while being recorded on video (two instances doing "from scratch" and two instances after AI pre-segmentation). This high-quality annotated dataset will be used for testing phase in 2025 Lighthouse challenge and will be publicly released at the completion of the challenge. The 2025 Lighthouse challenge will also release the 2023 and 2024 segmented datasets that were annotated using an established pipeline of pre-segmentation, student annotation, two neuroradiologists checking, and one neuroradiologist finalizing the process. It builds upon its previous edition by including post-treatment cases in the dataset. Using these high-quality annotated datasets, the 2025 Lighthouse challenge plans to test benchmark algorithms for automated segmentation of pre-and post-treatment brain metastases (BM), trained on diverse and multi-institutional datasets of MRI images obtained from patients with brain metastases.
The 2024 Brain Tumor Segmentation (BraTS) Challenge: Glioma Segmentation on Post-treatment MRI
May 28, 2024
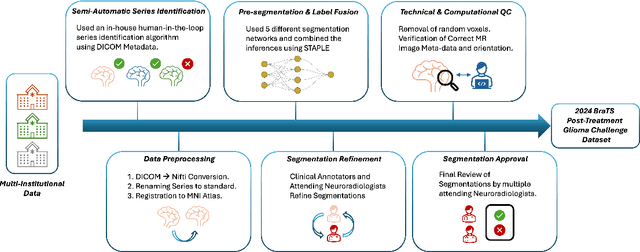
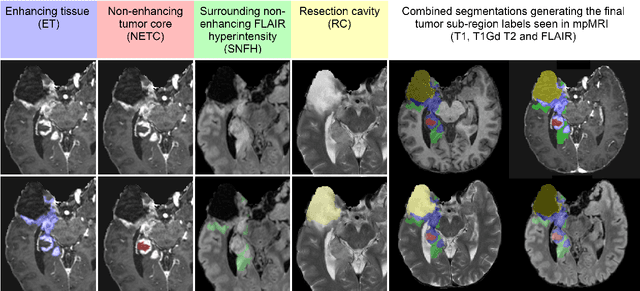
Abstract:Gliomas are the most common malignant primary brain tumors in adults and one of the deadliest types of cancer. There are many challenges in treatment and monitoring due to the genetic diversity and high intrinsic heterogeneity in appearance, shape, histology, and treatment response. Treatments include surgery, radiation, and systemic therapies, with magnetic resonance imaging (MRI) playing a key role in treatment planning and post-treatment longitudinal assessment. The 2024 Brain Tumor Segmentation (BraTS) challenge on post-treatment glioma MRI will provide a community standard and benchmark for state-of-the-art automated segmentation models based on the largest expert-annotated post-treatment glioma MRI dataset. Challenge competitors will develop automated segmentation models to predict four distinct tumor sub-regions consisting of enhancing tissue (ET), surrounding non-enhancing T2/fluid-attenuated inversion recovery (FLAIR) hyperintensity (SNFH), non-enhancing tumor core (NETC), and resection cavity (RC). Models will be evaluated on separate validation and test datasets using standardized performance metrics utilized across the BraTS 2024 cluster of challenges, including lesion-wise Dice Similarity Coefficient and Hausdorff Distance. Models developed during this challenge will advance the field of automated MRI segmentation and contribute to their integration into clinical practice, ultimately enhancing patient care.
Random Bundle: Brain Metastases Segmentation Ensembling through Annotation Randomization
Feb 23, 2020



Abstract:We introduce a novel ensembling method, Random Bundle (RB), that improves performance for brain metastases segmentation. We create our ensemble by training each network on our dataset with 50% of our annotated lesions censored out. We also apply a lopsided bootstrap loss to recover performance after inducing an in silico 50% false negative rate and make our networks more sensitive. We improve our network detection of lesions's mAP value by 39% and more than triple the sensitivity at 80% precision. We also show slight improvements in segmentation quality through DICE score. Further, RB ensembling improves performance over baseline by a larger margin than a variety of popular ensembling strategies. Finally, we show that RB ensembling is computationally efficient by comparing its performance to a single network when both systems are constrained to have the same compute.
Brain Metastasis Segmentation Network Trained with Robustness to Annotations with Multiple False Negatives
Jan 26, 2020



Abstract:Deep learning has proven to be an essential tool for medical image analysis. However, the need for accurately labeled input data, often requiring time- and labor-intensive annotation by experts, is a major limitation to the use of deep learning. One solution to this challenge is to allow for use of coarse or noisy labels, which could permit more efficient and scalable labeling of images. In this work, we develop a lopsided loss function based on entropy regularization that assumes the existence of a nontrivial false negative rate in the target annotations. Starting with a carefully annotated brain metastasis lesion dataset, we simulate data with false negatives by (1) randomly censoring the annotated lesions and (2) systematically censoring the smallest lesions. The latter better models true physician error because smaller lesions are harder to notice than the larger ones. Even with a simulated false negative rate as high as 50%, applying our loss function to randomly censored data preserves maximum sensitivity at 97% of the baseline with uncensored training data, compared to just 10% for a standard loss function. For the size-based censorship, performance is restored from 17% with the current standard to 88% with our lopsided bootstrap loss. Our work will enable more efficient scaling of the image labeling process, in parallel with other approaches on creating more efficient user interfaces and tools for annotation.
Handling Missing MRI Input Data in Deep Learning Segmentation of Brain Metastases: A Multi-Center Study
Dec 27, 2019



Abstract:The purpose was to assess the clinical value of a novel DropOut model for detecting and segmenting brain metastases, in which a neural network is trained on four distinct MRI sequences using an input dropout layer, thus simulating the scenario of missing MRI data by training on the full set and all possible subsets of the input data. This retrospective, multi-center study, evaluated 165 patients with brain metastases. A deep learning based segmentation model for automatic segmentation of brain metastases, named DropOut, was trained on multi-sequence MRI from 100 patients, and validated/tested on 10/55 patients. The segmentation results were compared with the performance of a state-of-the-art DeepLabV3 model. The MR sequences in the training set included pre- and post-gadolinium (Gd) T1-weighted 3D fast spin echo, post-Gd T1-weighted inversion recovery (IR) prepped fast spoiled gradient echo, and 3D fluid attenuated inversion recovery (FLAIR), whereas the test set did not include the IR prepped image-series. The ground truth were established by experienced neuroradiologists. The results were evaluated using precision, recall, Dice score, and receiver operating characteristics (ROC) curve statistics, while the Wilcoxon rank sum test was used to compare the performance of the two neural networks. The area under the ROC curve (AUC), averaged across all test cases, was 0.989+-0.029 for the DropOut model and 0.989+-0.023 for the DeepLabV3 model (p=0.62). The DropOut model showed a significantly higher Dice score compared to the DeepLabV3 model (0.795+-0.105 vs. 0.774+-0.104, p=0.017), and a significantly lower average false positive rate of 3.6/patient vs. 7.0/patient (p<0.001) using a 10mm3 lesion-size limit. The DropOut model may facilitate accurate detection and segmentation of brain metastases on a multi-center basis, even when the test cohort is missing MRI input data.
MRI Pulse Sequence Integration for Deep-Learning Based Brain Metastasis Segmentation
Dec 18, 2019



Abstract:Magnetic resonance (MR) imaging is an essential diagnostic tool in clinical medicine. Recently, a variety of deep learning methods have been applied to segmentation tasks in medical images, with promising results for computer-aided diagnosis. For MR images, effectively integrating different pulse sequences is important to optimize performance. However, the best way to integrate different pulse sequences remains unclear. In this study, we evaluate multiple architectural features and characterize their effects in the task of metastasis segmentation. Specifically, we consider (1) different pulse sequence integration schemas, (2) different modes of weight sharing for parallel network branches, and (3) a new approach for enabling robustness to missing pulse sequences. We find that levels of integration and modes of weight sharing that favor low variance work best in our regime of small data (n = 100). By adding an input-level dropout layer, we could preserve the overall performance of these networks while allowing for inference on inputs with missing pulse sequence. We illustrate not only the generalizability of the network but also the utility of this robustness when applying the trained model to data from a different center, which does not use the same pulse sequences. Finally, we apply network visualization methods to better understand which input features are most important for network performance. Together, these results provide a framework for building networks with enhanced robustness to missing data while maintaining comparable performance in medical imaging applications.
Deep Learning Enables Automatic Detection and Segmentation of Brain Metastases on Multi-Sequence MRI
Mar 18, 2019



Abstract:Detecting and segmenting brain metastases is a tedious and time-consuming task for many radiologists, particularly with the growing use of multi-sequence 3D imaging. This study demonstrates automated detection and segmentation of brain metastases on multi-sequence MRI using a deep learning approach based on a fully convolution neural network (CNN). In this retrospective study, a total of 156 patients with brain metastases from several primary cancers were included. Pre-therapy MR images (1.5T and 3T) included pre- and post-gadolinium T1-weighted 3D fast spin echo, post-gadolinium T1-weighted 3D axial IR-prepped FSPGR, and 3D fluid attenuated inversion recovery. The ground truth was established by manual delineation by two experienced neuroradiologists. CNN training/development was performed using 100 and 5 patients, respectively, with a 2.5D network based on a GoogLeNet architecture. The results were evaluated in 51 patients, equally separated into those with few (1-3), multiple (4-10), and many (>10) lesions. Network performance was evaluated using precision, recall, Dice/F1 score, and ROC-curve statistics. For an optimal probability threshold, detection and segmentation performance was assessed on a per metastasis basis. The area under the ROC-curve (AUC), averaged across all patients, was 0.98. The AUC in the subgroups was 0.99, 0.97, and 0.97 for patients having 1-3, 4-10, and >10 metastases, respectively. Using an average optimal probability threshold determined by the development set, precision, recall, and Dice-score were 0.79, 0.53, and 0.79, respectively. At the same probability threshold, the network showed an average false positive rate of 8.3/patient (no lesion-size limit) and 3.4/patient (10 mm3 lesion size limit). In conclusion, a deep learning approach using multi-sequence MRI can aid in the detection and segmentation of brain metastases.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge