M. Ethan MacDonald
Genetic Influences on Brain Aging: Analyzing Sex Differences in the UK Biobank using Structural MRI
May 25, 2025Abstract:Brain aging trajectories differ between males and females, yet the genetic factors underlying these differences remain underexplored. Using structural MRI and genotyping data from 40,940 UK Biobank participants (aged 45-83), we computed Brain Age Gap Estimates (BrainAGE) for total brain, hippocampal, and ventricular volumes. We conducted sex-stratified genome-wide association studies (GWAS) and Post-GWAS analyses to identify genetic variants associated with accelerated brain aging. Distinct gene sets emerged by sex: in females, neurotransmitter transport and mitochondrial stress response genes were implicated; in males, immune and inflammation-related genes dominated. Shared genes, including GMNC and OSTN, were consistently linked to brain volumes across sexes, suggesting core roles in neurostructural maintenance. Tissue expression analyses revealed sex-specific enrichment in pathways tied to neurodegeneration. These findings highlight the importance of sex-stratified approaches in aging research and suggest genetic targets for personalized interventions against age-related cognitive decline.
* 7 pages, 5 figures, conference
Direct Estimation of Pediatric Heart Rate Variability from BOLD-fMRI: A Machine Learning Approach Using Dynamic Connectivity
Feb 10, 2025Abstract:In many pediatric fMRI studies, cardiac signals are often missing or of poor quality. A tool to extract Heart Rate Variation (HRV) waveforms directly from fMRI data, without the need for peripheral recording devices, would be highly beneficial. We developed a machine learning framework to accurately reconstruct HRV for pediatric applications. A hybrid model combining one-dimensional Convolutional Neural Networks (1D-CNN) and Gated Recurrent Units (GRU) analyzed BOLD signals from 628 ROIs, integrating past and future data. The model achieved an 8% improvement in HRV accuracy, as evidenced by enhanced performance metrics. This approach eliminates the need for peripheral photoplethysmography devices, reduces costs, and simplifies procedures in pediatric fMRI. Additionally, it improves the robustness of pediatric fMRI studies, which are more sensitive to physiological and developmental variations than those in adults.
Three-Dimensional Amyloid-Beta PET Synthesis from Structural MRI with Conditional Generative Adversarial Networks
May 03, 2024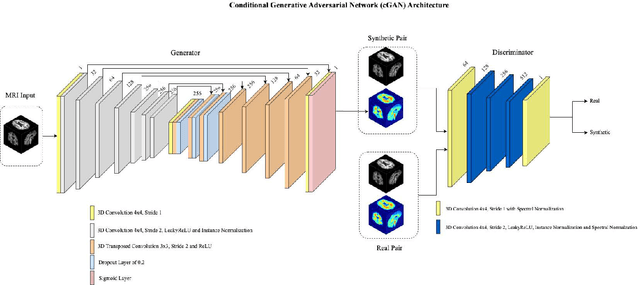
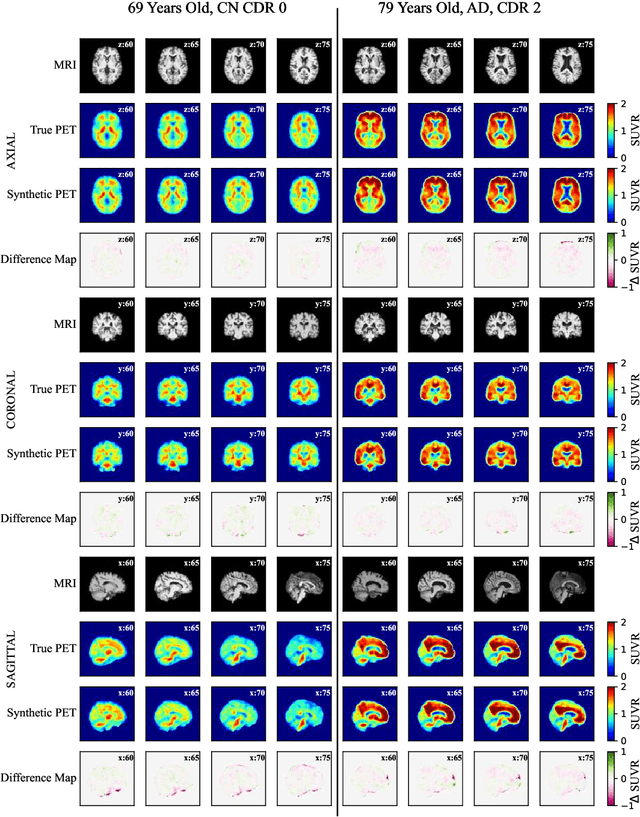
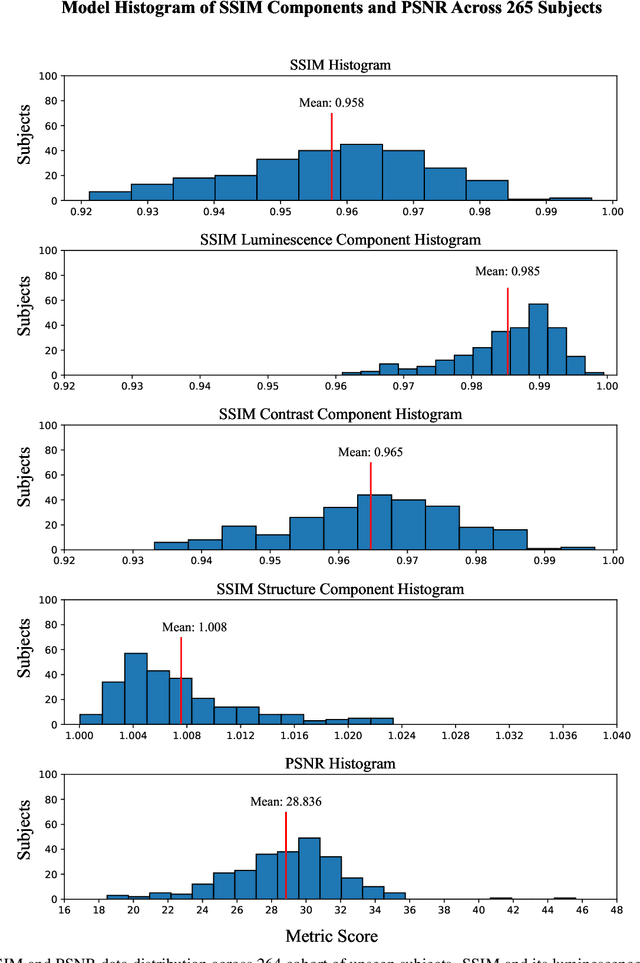

Abstract:Motivation: Alzheimer's Disease hallmarks include amyloid-beta deposits and brain atrophy, detectable via PET and MRI scans, respectively. PET is expensive, invasive and exposes patients to ionizing radiation. MRI is cheaper, non-invasive, and free from ionizing radiation but limited to measuring brain atrophy. Goal: To develop an 3D image translation model that synthesizes amyloid-beta PET images from T1-weighted MRI, exploiting the known relationship between amyloid-beta and brain atrophy. Approach: The model was trained on 616 PET/MRI pairs and validated with 264 pairs. Results: The model synthesized amyloid-beta PET images from T1-weighted MRI with high-degree of similarity showing high SSIM and PSNR metrics (SSIM>0.95&PSNR=28). Impact: Our model proves the feasibility of synthesizing amyloid-beta PET images from structural MRI ones, significantly enhancing accessibility for large-cohort studies and early dementia detection, while also reducing cost, invasiveness, and radiation exposure.
Machine Learning-based Estimation of Respiratory Fluctuations in a Healthy Adult Population using BOLD fMRI and Head Motion Parameters
Apr 30, 2024



Abstract:Motivation: In many fMRI studies, respiratory signals are often missing or of poor quality. Therefore, it could be highly beneficial to have a tool to extract respiratory variation (RV) waveforms directly from fMRI data without the need for peripheral recording devices. Goal(s): Investigate the hypothesis that head motion parameters contain valuable information regarding respiratory patter, which can help machine learning algorithms estimate the RV waveform. Approach: This study proposes a CNN model for reconstruction of RV waveforms using head motion parameters and BOLD signals. Results: This study showed that combining head motion parameters with BOLD signals enhances RV waveform estimation. Impact: It is expected that application of the proposed method will lower the cost of fMRI studies, reduce complexity, and decrease the burden on participants as they will not be required to wear a respiratory bellows.
A voxel-level approach to brain age prediction: A method to assess regional brain aging
Oct 17, 2023



Abstract:Brain aging is a regional phenomenon, a facet that remains relatively under-explored within the realm of brain age prediction research using machine learning methods. Voxel-level predictions can provide localized brain age estimates that can provide granular insights into the regional aging processes. This is essential to understand the differences in aging trajectories in healthy versus diseased subjects. In this work, a deep learning-based multitask model is proposed for voxel-level brain age prediction from T1-weighted magnetic resonance images. The proposed model outperforms the models existing in the literature and yields valuable clinical insights when applied to both healthy and diseased populations. Regional analysis is performed on the voxel-level brain age predictions to understand aging trajectories of known anatomical regions in the brain and show that there exist disparities in regional aging trajectories of healthy subjects compared to ones with underlying neurological disorders such as Dementia and more specifically, Alzheimer's disease. Our code is available at https://github.com/nehagianchandani/Voxel-level-brain-age-prediction.
Amyloid-Beta Axial Plane PET Synthesis from Structural MRI: An Image Translation Approach for Screening Alzheimer's Disease
Sep 01, 2023
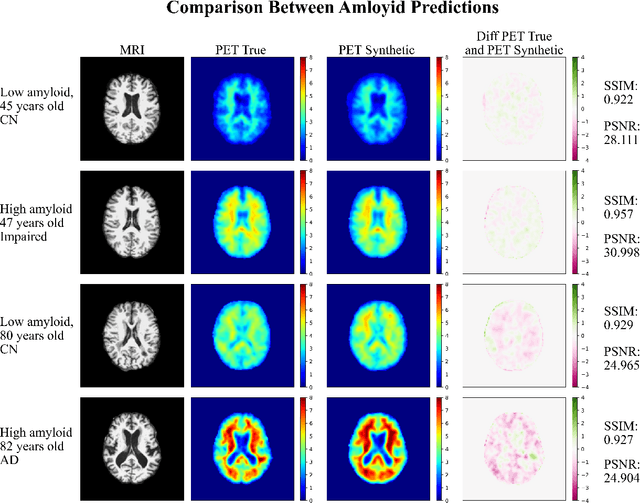

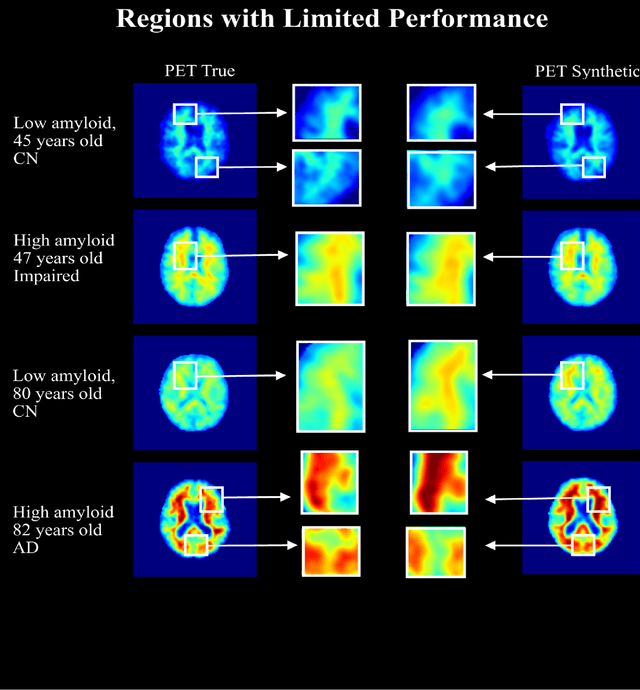
Abstract:In this work, an image translation model is implemented to produce synthetic amyloid-beta PET images from structural MRI that are quantitatively accurate. Image pairs of amyloid-beta PET and structural MRI were used to train the model. We found that the synthetic PET images could be produced with a high degree of similarity to truth in terms of shape, contrast and overall high SSIM and PSNR. This work demonstrates that performing structural to quantitative image translation is feasible to enable the access amyloid-beta information from only MRI.
The Effect of Epidemiological Cohort Creation on the Machine Learning Prediction of Homelessness and Police Interaction Outcomes Using Administrative Health Care Data
Jul 20, 2023



Abstract:Background: Mental illness can lead to adverse outcomes such as homelessness and police interaction and understanding of the events leading up to these adverse outcomes is important. Predictive models may help identify individuals at risk of such adverse outcomes. Using a fixed observation window cohort with logistic regression (LR) or machine learning (ML) models can result in lower performance when compared with adaptive and parcellated windows. Method: An administrative healthcare dataset was used, comprising of 240,219 individuals in Calgary, Alberta, Canada who were diagnosed with addiction or mental health (AMH) between April 1, 2013, and March 31, 2018. The cohort was followed for 2 years to identify factors associated with homelessness and police interactions. To understand the benefit of flexible windows to predictive models, an alternative cohort was created. Then LR and ML models, including random forests (RF), and extreme gradient boosting (XGBoost) were compared in the two cohorts. Results: Among 237,602 individuals, 0.8% (1,800) experienced first homelessness, while 0.32% (759) reported initial police interaction among 237,141 individuals. Male sex (AORs: H=1.51, P=2.52), substance disorder (AORs: H=3.70, P=2.83), psychiatrist visits (AORs: H=1.44, P=1.49), and drug abuse (AORs: H=2.67, P=1.83) were associated with initial homelessness (H) and police interaction (P). XGBoost showed superior performance using the flexible method (sensitivity =91%, AUC =90% for initial homelessness, and sensitivity =90%, AUC=89% for initial police interaction) Conclusion: This study identified key features associated with initial homelessness and police interaction and demonstrated that flexible windows can improve predictive modeling.
Denoising Simulated Low-Field MRI (70mT) using Denoising Autoencoders (DAE) and Cycle-Consistent Generative Adversarial Networks (Cycle-GAN)
Jul 12, 2023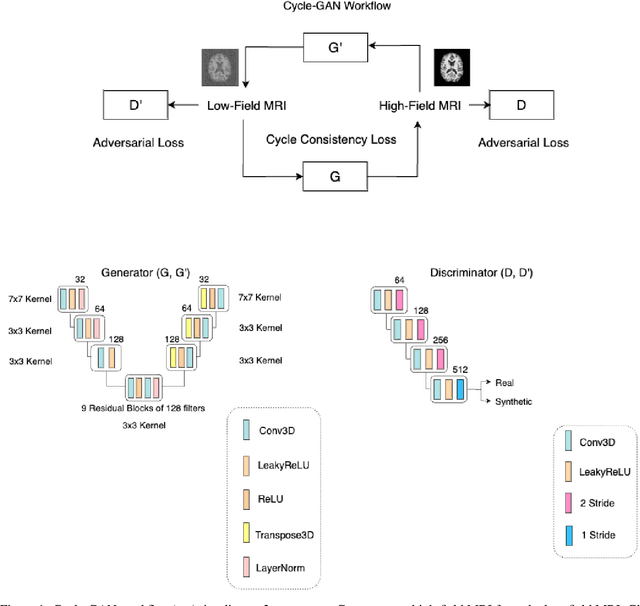
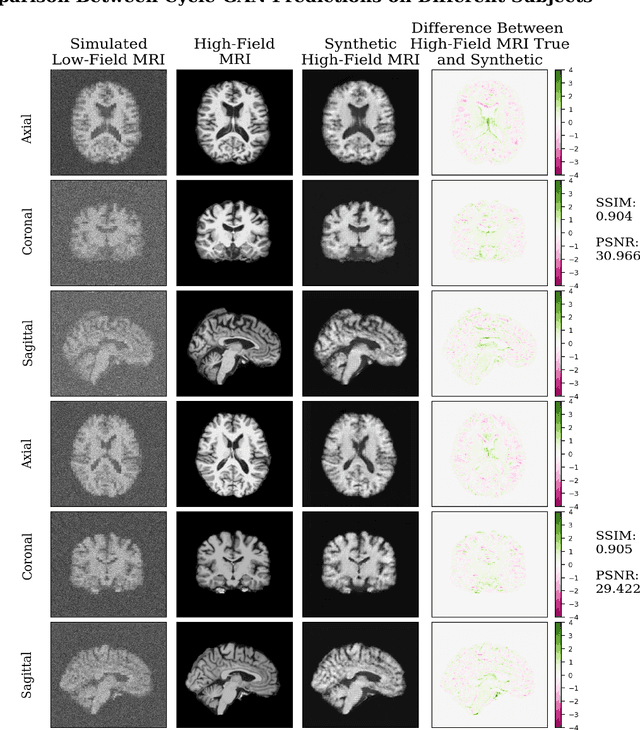
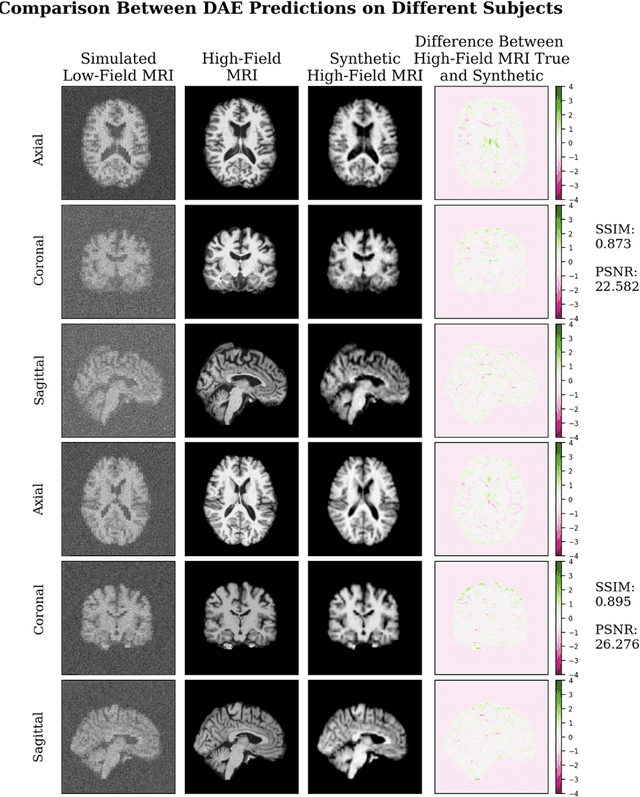
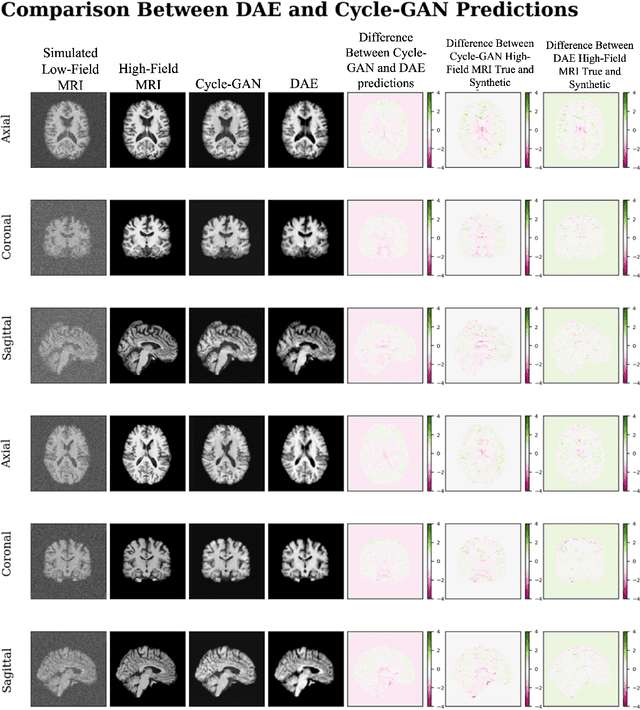
Abstract:In this work, a denoising Cycle-GAN (Cycle Consistent Generative Adversarial Network) is implemented to yield high-field, high resolution, high signal-to-noise ratio (SNR) Magnetic Resonance Imaging (MRI) images from simulated low-field, low resolution, low SNR MRI images. Resampling and additive Rician noise were used to simulate low-field MRI. Images were utilized to train a Denoising Autoencoder (DAE) and a Cycle-GAN, with paired and unpaired cases. Both networks were evaluated using SSIM and PSNR image quality metrics. This work demonstrates the use of a generative deep learning model that can outperform classical DAEs to improve low-field MRI images and does not require image pairs.
Using BOLD-fMRI to Compute the Respiration Volume per Time and Respiration Variation with Convolutional Neural Networks in the Human Connectome Development Cohort
Jul 03, 2023



Abstract:In many fMRI studies, respiratory signals are unavailable or do not have acceptable quality. Consequently, the direct removal of low-frequency respiratory variations from BOLD signals is not possible. This study proposes a one-dimensional CNN model for reconstruction of two respiratory measures, RV and RVT. Results show that a CNN can capture informative features from resting BOLD signals and reconstruct realistic RV and RVT timeseries. It is expected that application of the proposed method will lower the cost of fMRI studies, reduce complexity, and decrease the burden on participants as they will not be required to wear a respiratory bellows.
Bidirectional Modeling and Analysis of Brain Aging with Normalizing Flows
Nov 26, 2020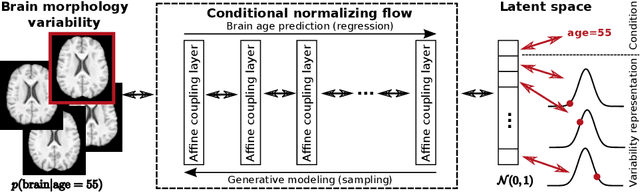
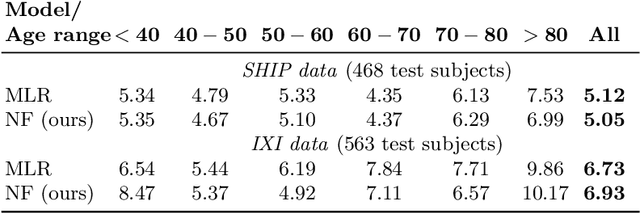
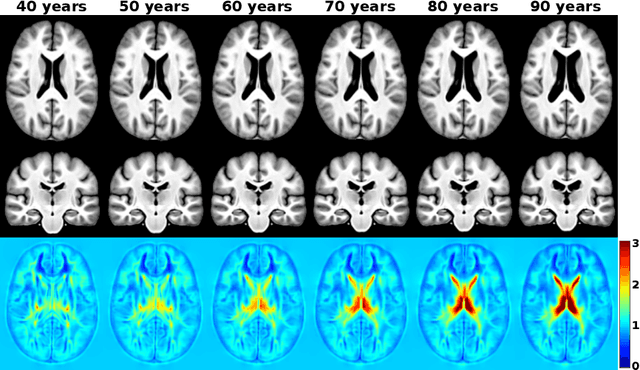
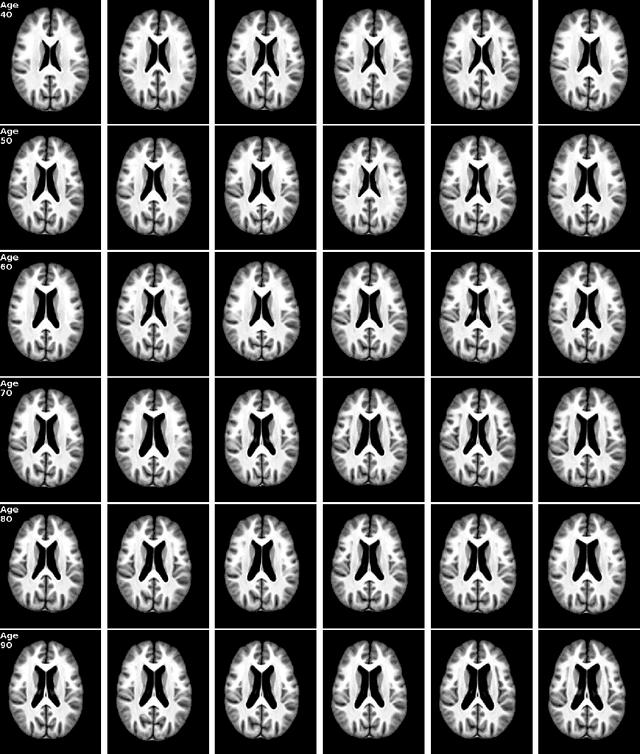
Abstract:Brain aging is a widely studied longitudinal process throughout which the brain undergoes considerable morphological changes and various machine learning approaches have been proposed to analyze it. Within this context, brain age prediction from structural MR images and age-specific brain morphology template generation are two problems that have attracted much attention. While most approaches tackle these tasks independently, we assume that they are inverse directions of the same functional bidirectional relationship between a brain's morphology and an age variable. In this paper, we propose to model this relationship with a single conditional normalizing flow, which unifies brain age prediction and age-conditioned generative modeling in a novel way. In an initial evaluation of this idea, we show that our normalizing flow brain aging model can accurately predict brain age while also being able to generate age-specific brain morphology templates that realistically represent the typical aging trend in a healthy population. This work is a step towards unified modeling of functional relationships between 3D brain morphology and clinical variables of interest with powerful normalizing flows.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge