Matthias Wilms
Cumming School of Medicine, Department of Radiology, University of Calgary, Calgary, Canada
MACAW: A Causal Generative Model for Medical Imaging
Dec 03, 2024



Abstract:Although deep learning techniques show promising results for many neuroimaging tasks in research settings, they have not yet found widespread use in clinical scenarios. One of the reasons for this problem is that many machine learning models only identify correlations between the input images and the outputs of interest, which can lead to many practical problems, such as encoding of uninformative biases and reduced explainability. Thus, recent research is exploring if integrating a priori causal knowledge into deep learning models is a potential avenue to identify these problems. This work introduces a new causal generative architecture named Masked Causal Flow (MACAW) for neuroimaging applications. Within this context, three main contributions are described. First, a novel approach that integrates complex causal structures into normalizing flows is proposed. Second, counterfactual prediction is performed to identify the changes in effect variables associated with a cause variable. Finally, an explicit Bayesian inference for classification is derived and implemented, providing an inherent uncertainty estimation. The feasibility of the proposed method was first evaluated using synthetic data and then using MRI brain data from more than 23000 participants of the UK biobank study. The evaluation results show that the proposed method can (1) accurately encode causal reasoning and generate counterfactuals highlighting the structural changes in the brain known to be associated with aging, (2) accurately predict a subject's age from a single 2D MRI slice, and (3) generate new samples assuming other values for subject-specific indicators such as age, sex, and body mass index. The code for a toy dataset is available at the following link: https://github.com/vibujithan/macaw-2D.git.
Trustworthy and Responsible AI for Human-Centric Autonomous Decision-Making Systems
Sep 02, 2024Abstract:Artificial Intelligence (AI) has paved the way for revolutionary decision-making processes, which if harnessed appropriately, can contribute to advancements in various sectors, from healthcare to economics. However, its black box nature presents significant ethical challenges related to bias and transparency. AI applications are hugely impacted by biases, presenting inconsistent and unreliable findings, leading to significant costs and consequences, highlighting and perpetuating inequalities and unequal access to resources. Hence, developing safe, reliable, ethical, and Trustworthy AI systems is essential. Our team of researchers working with Trustworthy and Responsible AI, part of the Transdisciplinary Scholarship Initiative within the University of Calgary, conducts research on Trustworthy and Responsible AI, including fairness, bias mitigation, reproducibility, generalization, interpretability, and authenticity. In this paper, we review and discuss the intricacies of AI biases, definitions, methods of detection and mitigation, and metrics for evaluating bias. We also discuss open challenges with regard to the trustworthiness and widespread application of AI across diverse domains of human-centric decision making, as well as guidelines to foster Responsible and Trustworthy AI models.
Towards objective and systematic evaluation of bias in medical imaging AI
Nov 03, 2023



Abstract:Artificial intelligence (AI) models trained using medical images for clinical tasks often exhibit bias in the form of disparities in performance between subgroups. Since not all sources of biases in real-world medical imaging data are easily identifiable, it is challenging to comprehensively assess how those biases are encoded in models, and how capable bias mitigation methods are at ameliorating performance disparities. In this article, we introduce a novel analysis framework for systematically and objectively investigating the impact of biases in medical images on AI models. We developed and tested this framework for conducting controlled in silico trials to assess bias in medical imaging AI using a tool for generating synthetic magnetic resonance images with known disease effects and sources of bias. The feasibility is showcased by using three counterfactual bias scenarios to measure the impact of simulated bias effects on a convolutional neural network (CNN) classifier and the efficacy of three bias mitigation strategies. The analysis revealed that the simulated biases resulted in expected subgroup performance disparities when the CNN was trained on the synthetic datasets. Moreover, reweighing was identified as the most successful bias mitigation strategy for this setup, and we demonstrated how explainable AI methods can aid in investigating the manifestation of bias in the model using this framework. Developing fair AI models is a considerable challenge given that many and often unknown sources of biases can be present in medical imaging datasets. In this work, we present a novel methodology to objectively study the impact of biases and mitigation strategies on deep learning pipelines, which can support the development of clinical AI that is robust and responsible.
Bidirectional Modeling and Analysis of Brain Aging with Normalizing Flows
Nov 26, 2020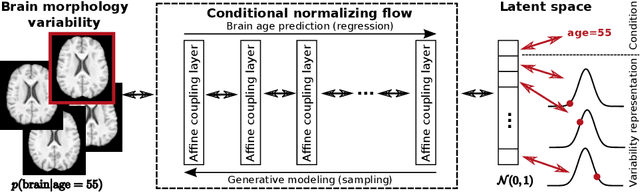
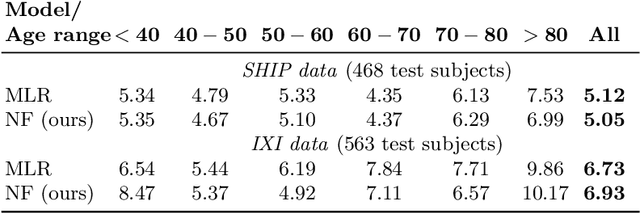
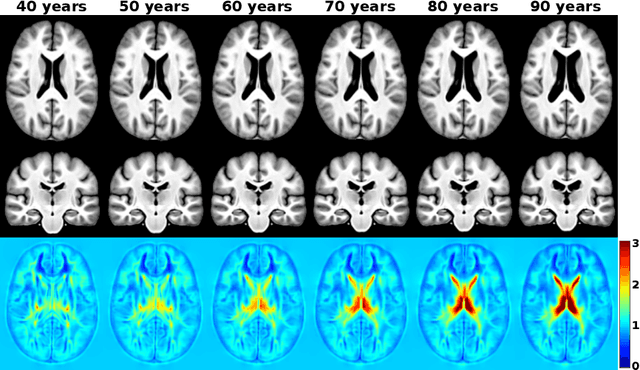
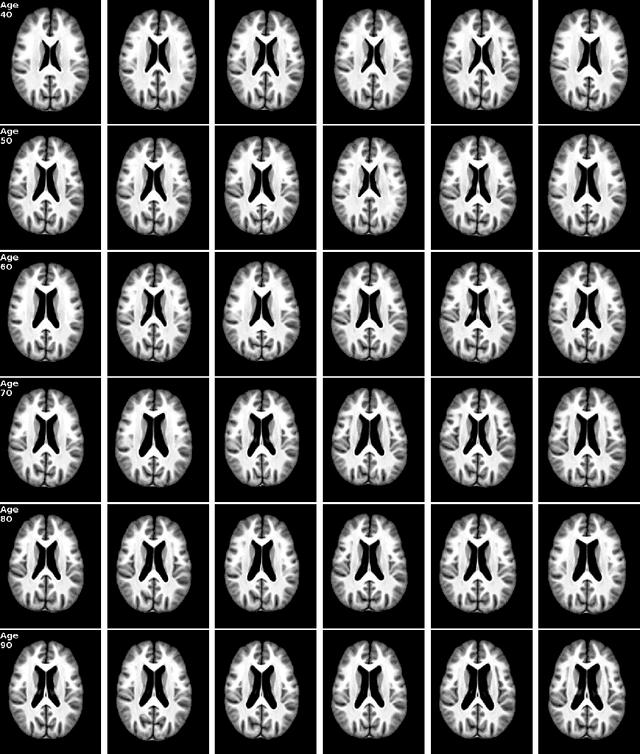
Abstract:Brain aging is a widely studied longitudinal process throughout which the brain undergoes considerable morphological changes and various machine learning approaches have been proposed to analyze it. Within this context, brain age prediction from structural MR images and age-specific brain morphology template generation are two problems that have attracted much attention. While most approaches tackle these tasks independently, we assume that they are inverse directions of the same functional bidirectional relationship between a brain's morphology and an age variable. In this paper, we propose to model this relationship with a single conditional normalizing flow, which unifies brain age prediction and age-conditioned generative modeling in a novel way. In an initial evaluation of this idea, we show that our normalizing flow brain aging model can accurately predict brain age while also being able to generate age-specific brain morphology templates that realistically represent the typical aging trend in a healthy population. This work is a step towards unified modeling of functional relationships between 3D brain morphology and clinical variables of interest with powerful normalizing flows.
Population-based Respiratory 4D Motion Atlas Construction and its Application for VR Simulations of Liver Punctures
Dec 22, 2017Abstract:Virtual reality (VR) training simulators of liver needle insertion in the hepatic area of breathing virtual patients currently need 4D data acquisitions as a prerequisite. Here, first a population-based breathing virtual patient 4D atlas can be built and second the requirement of a dose-relevant or expensive acquisition of a 4D data set for a new static 3D patient can be mitigated by warping the mean atlas motion. The breakthrough contribution of this work is the construction and reuse of population-based learned 4D motion models.
Interpatient Respiratory Motion Model Transfer for Virtual Reality Simulations of Liver Punctures
Aug 02, 2017

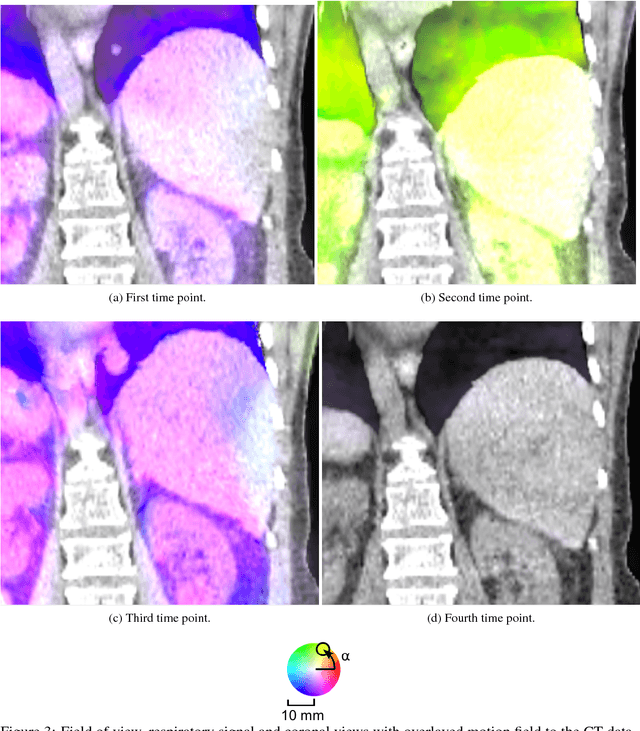

Abstract:Current virtual reality (VR) training simulators of liver punctures often rely on static 3D patient data and use an unrealistic (sinusoidal) periodic animation of the respiratory movement. Existing methods for the animation of breathing motion support simple mathematical or patient-specific, estimated breathing models. However with personalized breathing models for each new patient, a heavily dose relevant or expensive 4D data acquisition is mandatory for keyframe-based motion modeling. Given the reference 4D data, first a model building stage using linear regression motion field modeling takes place. Then the methodology shown here allows the transfer of existing reference respiratory motion models of a 4D reference patient to a new static 3D patient. This goal is achieved by using non-linear inter-patient registration to warp one personalized 4D motion field model to new 3D patient data. This cost- and dose-saving new method is shown here visually in a qualitative proof-of-concept study.
* World Society for Computer Graphics - WSCG 2017 publication, 9 pages, 5 figures, 1 movie online
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge