Leonhard F. Feiner
Propagation and Attribution of Uncertainty in Medical Imaging Pipelines
Sep 28, 2023Abstract:Uncertainty estimation, which provides a means of building explainable neural networks for medical imaging applications, have mostly been studied for single deep learning models that focus on a specific task. In this paper, we propose a method to propagate uncertainty through cascades of deep learning models in medical imaging pipelines. This allows us to aggregate the uncertainty in later stages of the pipeline and to obtain a joint uncertainty measure for the predictions of later models. Additionally, we can separately report contributions of the aleatoric, data-based, uncertainty of every component in the pipeline. We demonstrate the utility of our method on a realistic imaging pipeline that reconstructs undersampled brain and knee magnetic resonance (MR) images and subsequently predicts quantitative information from the images, such as the brain volume, or knee side or patient's sex. We quantitatively show that the propagated uncertainty is correlated with input uncertainty and compare the proportions of contributions of pipeline stages to the joint uncertainty measure.
Extended Graph Assessment Metrics for Graph Neural Networks
Jul 13, 2023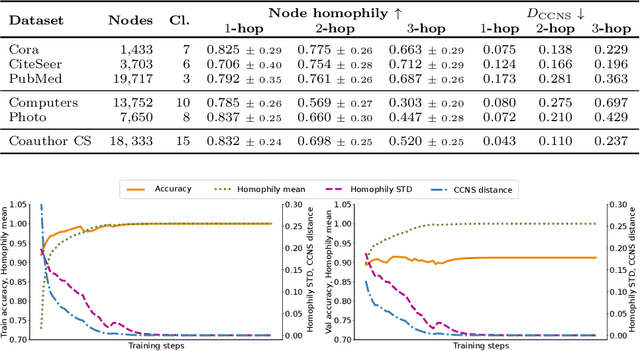
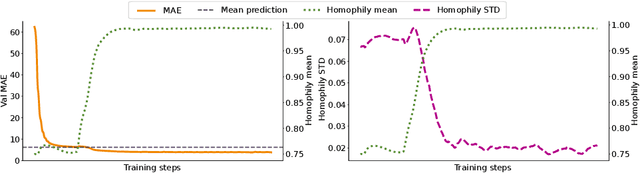
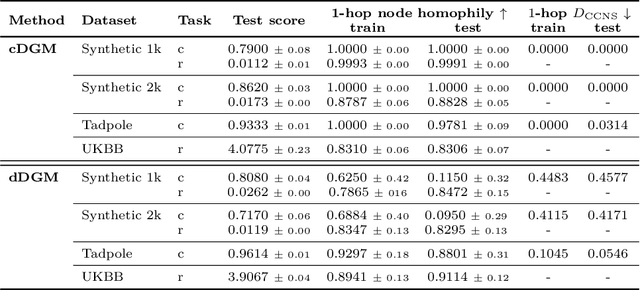
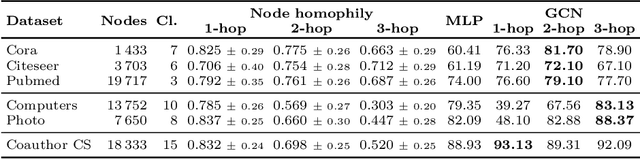
Abstract:When re-structuring patient cohorts into so-called population graphs, initially independent data points can be incorporated into one interconnected graph structure. This population graph can then be used for medical downstream tasks using graph neural networks (GNNs). The construction of a suitable graph structure is a challenging step in the learning pipeline that can have severe impact on model performance. To this end, different graph assessment metrics have been introduced to evaluate graph structures. However, these metrics are limited to classification tasks and discrete adjacency matrices, only covering a small subset of real-world applications. In this work, we introduce extended graph assessment metrics (GAMs) for regression tasks and continuous adjacency matrices. We focus on two GAMs in specific: \textit{homophily} and \textit{cross-class neighbourhood similarity} (CCNS). We extend the notion of GAMs to more than one hop, define homophily for regression tasks, as well as continuous adjacency matrices, and propose a light-weight CCNS distance for discrete and continuous adjacency matrices. We show the correlation of these metrics with model performance on different medical population graphs and under different learning settings.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge