Lang Wang
CAMEL2: Enhancing weakly supervised learning for histopathology images by incorporating the significance ratio
Oct 09, 2023Abstract:Histopathology image analysis plays a crucial role in cancer diagnosis. However, training a clinically applicable segmentation algorithm requires pathologists to engage in labour-intensive labelling. In contrast, weakly supervised learning methods, which only require coarse-grained labels at the image level, can significantly reduce the labeling efforts. Unfortunately, while these methods perform reasonably well in slide-level prediction, their ability to locate cancerous regions, which is essential for many clinical applications, remains unsatisfactory. Previously, we proposed CAMEL, which achieves comparable results to those of fully supervised baselines in pixel-level segmentation. However, CAMEL requires 1,280x1,280 image-level binary annotations for positive WSIs. Here, we present CAMEL2, by introducing a threshold of the cancerous ratio for positive bags, it allows us to better utilize the information, consequently enabling us to scale up the image-level setting from 1,280x1,280 to 5,120x5,120 while maintaining the accuracy. Our results with various datasets, demonstrate that CAMEL2, with the help of 5,120x5,120 image-level binary annotations, which are easy to annotate, achieves comparable performance to that of a fully supervised baseline in both instance- and slide-level classifications.
Deeply Supervised Layer Selective Attention Network: Towards Label-Efficient Learning for Medical Image Classification
Sep 28, 2022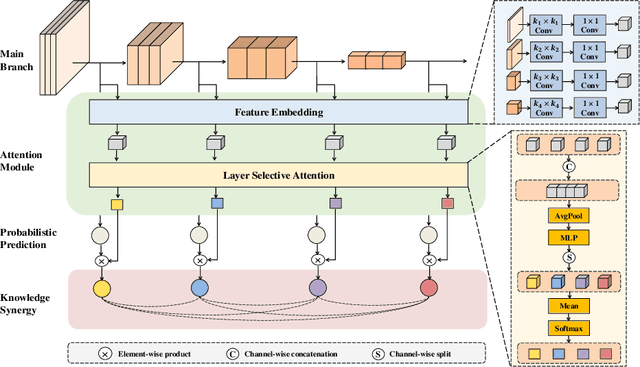
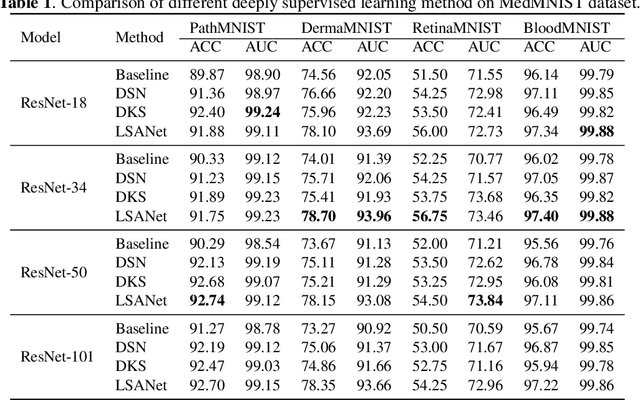
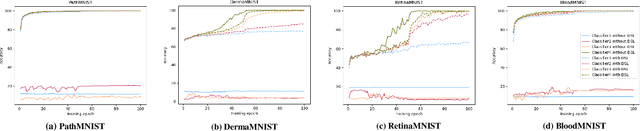
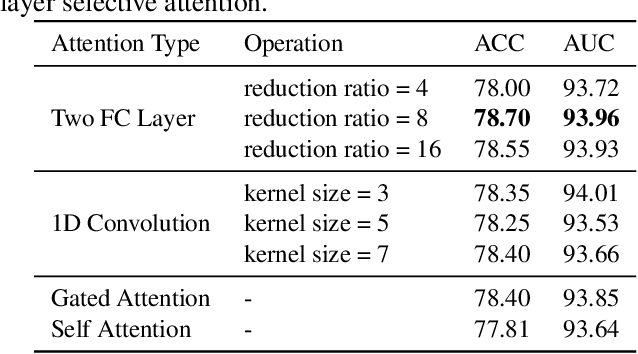
Abstract:Labeling medical images depends on professional knowledge, making it difficult to acquire large amount of annotated medical images with high quality in a short time. Thus, making good use of limited labeled samples in a small dataset to build a high-performance model is the key to medical image classification problem. In this paper, we propose a deeply supervised Layer Selective Attention Network (LSANet), which comprehensively uses label information in feature-level and prediction-level supervision. For feature-level supervision, in order to better fuse the low-level features and high-level features, we propose a novel visual attention module, Layer Selective Attention (LSA), to focus on the feature selection of different layers. LSA introduces a weight allocation scheme which can dynamically adjust the weighting factor of each auxiliary branch during the whole training process to further enhance deeply supervised learning and ensure its generalization. For prediction-level supervision, we adopt the knowledge synergy strategy to promote hierarchical information interactions among all supervision branches via pairwise knowledge matching. Using the public dataset, MedMNIST, which is a large-scale benchmark for biomedical image classification covering diverse medical specialties, we evaluate LSANet on multiple mainstream CNN architectures and various visual attention modules. The experimental results show the substantial improvements of our proposed method over its corresponding counterparts, demonstrating that LSANet can provide a promising solution for label-efficient learning in the field of medical image classification.
Automated Scoring System of HER2 in Pathological Images under the Microscope
Oct 21, 2021

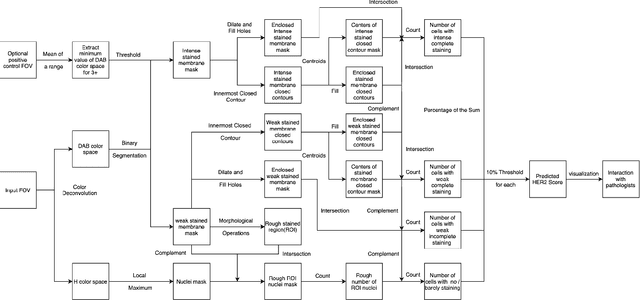

Abstract:Breast cancer is the most common cancer among women worldwide. The human epidermal growth factor receptor 2(HER2) with immunohistochemical(IHC) is widely used for pathological evaluation to provide the appropriate therapy for patients with breast cancer. However, the deficiency of pathologists is extremely significant in the current society, and visual diagnosis of the HER2 overexpression is subjective and susceptible to inter-observer variation. Recently, with the rapid development of artificial intelligence(AI) in disease diagnosis, several automated HER2 scoring methods using traditional computer vision or machine learning methods indicate the improvement of the HER2 diagnostic accuracy, but the unreasonable interpretation in pathology, as well as the expensive and ethical issues for annotation, make these methods still have a long way to deploy in hospitals to ease pathologists' burden in real. In this paper, we propose a HER2 automated scoring system that strictly follows the HER2 scoring guidelines simulating the real workflow of HER2 scores diagnosis by pathologists. Unlike the previous work, our method takes the positive control of HER2 into account to make sure the assay performance for each slide, eliminating work for repeated comparison and checking for the current field of view(FOV) and positive control FOV, especially for the borderline cases. Besides, for each selected FOV under the microscope, our system provides real-time HER2 scores analysis and visualizations of the membrane staining intensity and completeness corresponding with the cell classification. Our rigorous workflow along with the flexible interactive adjustion in demand substantially assists pathologists to finish the HER2 diagnosis faster and improves the robustness and accuracy. The proposed system will be embedded in our Thorough Eye platform for deployment in hospitals.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge