José V. Manjón
Ultra-high resolution multimodal MRI dense labelled holistic brain atlas
Jan 28, 2025



Abstract:In this paper, we introduce holiAtlas, a holistic, multimodal and high-resolution human brain atlas. This atlas covers different levels of details of the human brain anatomy, from the organ to the substructure level, using a new dense labelled protocol generated from the fusion of multiple local protocols at different scales. This atlas has been constructed averaging images and segmentations of 75 healthy subjects from the Human Connectome Project database. Specifically, MR images of T1, T2 and WMn (White Matter nulled) contrasts at 0.125 $mm^{3}$ resolution that were nonlinearly registered and averaged using symmetric group-wise normalisation to construct the atlas. At the finest level, the holiAtlas protocol has 350 different labels derived from 10 different delineation protocols. These labels were grouped at different scales to provide a holistic view of the brain at different levels in a coherent and consistent manner. This multiscale and multimodal atlas can be used for the development of new ultra-high resolution segmentation methods that can potentially leverage the early detection of neurological disorders.
DeepCERES: A Deep learning method for cerebellar lobule segmentation using ultra-high resolution multimodal MRI
Jan 23, 2024Abstract:This paper introduces a novel multimodal and high-resolution human brain cerebellum lobule segmentation method. Unlike current tools that operate at standard resolution ($1 \text{ mm}^{3}$) or using mono-modal data, the proposed method improves cerebellum lobule segmentation through the use of a multimodal and ultra-high resolution ($0.125 \text{ mm}^{3}$) training dataset. To develop the method, first, a database of semi-automatically labelled cerebellum lobules was created to train the proposed method with ultra-high resolution T1 and T2 MR images. Then, an ensemble of deep networks has been designed and developed, allowing the proposed method to excel in the complex cerebellum lobule segmentation task, improving precision while being memory efficient. Notably, our approach deviates from the traditional U-Net model by exploring alternative architectures. We have also integrated deep learning with classical machine learning methods incorporating a priori knowledge from multi-atlas segmentation, which improved precision and robustness. Finally, a new online pipeline, named DeepCERES, has been developed to make available the proposed method to the scientific community requiring as input only a single T1 MR image at standard resolution.
DeepThalamus: A novel deep learning method for automatic segmentation of brain thalamic nuclei from multimodal ultra-high resolution MRI
Jan 15, 2024Abstract:The implication of the thalamus in multiple neurological pathologies makes it a structure of interest for volumetric analysis. In the present work, we have designed and implemented a multimodal volumetric deep neural network for the segmentation of thalamic nuclei at ultra-high resolution (0.125 mm3). Current tools either operate at standard resolution (1 mm3) or use monomodal data. To achieve the proposed objective, first, a database of semiautomatically segmented thalamic nuclei was created using ultra-high resolution T1, T2 and White Matter nulled (WMn) images. Then, a novel Deep learning based strategy was designed to obtain the automatic segmentations and trained to improve its robustness and accuaracy using a semisupervised approach. The proposed method was compared with a related state-of-the-art method showing competitive results both in terms of segmentation quality and efficiency. To make the proposed method fully available to the scientific community, a full pipeline able to work with monomodal standard resolution T1 images is also proposed.
Multi-scale Graph-based Grading for Alzheimer's Disease Prediction
Jul 15, 2019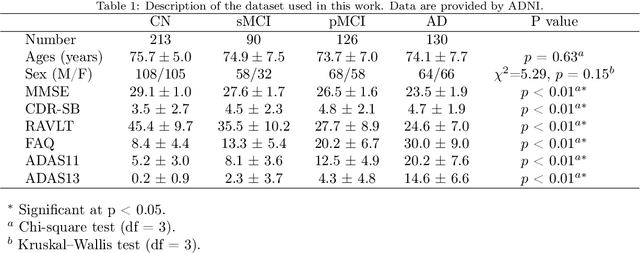
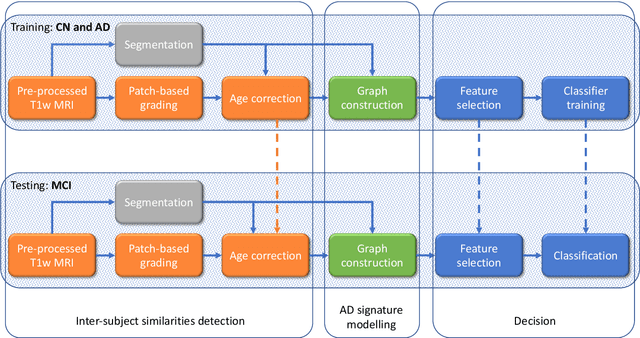
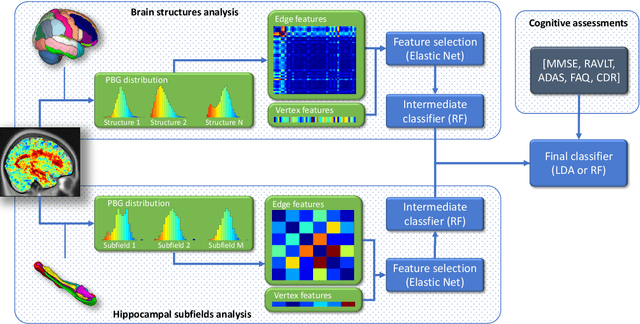
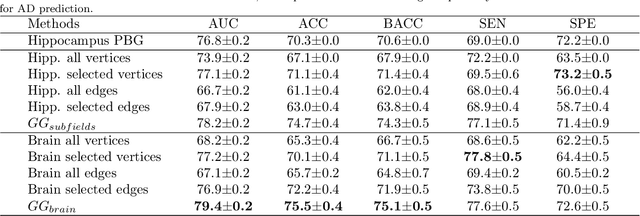
Abstract:The prediction of subjects with mild cognitive impairment (MCI) who will progress to Alzheimer's disease (AD) is clinically relevant, and may above all have a significant impact on accelerate the development of new treatments. In this paper, we present a new MRI-based biomarker that enables us to predict conversion of MCI subjects to AD accurately. In order to better capture the AD signature, we introduce two main contributions. First, we present a new graph-based grading framework to combine inter-subject similarity features and intra-subject variability features. This framework involves patch-based grading of anatomical structures and graph-based modeling of structure alteration relationships. Second, we propose an innovative multiscale brain analysis to capture alterations caused by AD at different anatomical levels. Based on a cascade of classifiers, this multiscale approach enables the analysis of alterations of whole brain structures and hippocampus subfields at the same time. During our experiments using the ADNI-1 dataset, the proposed multiscale graph-based grading method obtained an area under the curve (AUC) of 81% to predict conversion of MCI subjects to AD within three years. Moreover, when combined with cognitive scores, the proposed method obtained 85% of AUC. These results are competitive in comparison to state-of-the-art methods evaluated on the same dataset.
An Optimized PatchMatch for Multi-scale and Multi-feature Label Fusion
Mar 17, 2019


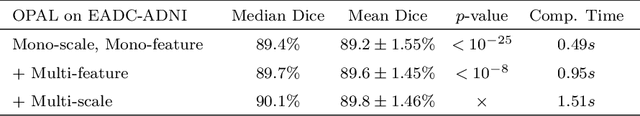
Abstract:Automatic segmentation methods are important tools for quantitative analysis of Magnetic Resonance Images (MRI). Recently, patch-based label fusion approaches have demonstrated state-of-the-art segmentation accuracy. In this paper, we introduce a new patch-based label fusion framework to perform segmentation of anatomical structures. The proposed approach uses an Optimized PAtchMatch Label fusion (OPAL) strategy that drastically reduces the computation time required for the search of similar patches. The reduced computation time of OPAL opens the way for new strategies and facilitates processing on large databases. In this paper, we investigate new perspectives offered by OPAL, by introducing a new multi-scale and multi-feature framework. During our validation on hippocampus segmentation we use two datasets: young adults in the ICBM cohort and elderly adults in the EADC-ADNI dataset. For both, OPAL is compared to state-of-the-art methods. Results show that OPAL obtained the highest median Dice coefficient (89.9% for ICBM and 90.1% for EADC-ADNI). Moreover, in both cases, OPAL produced a segmentation accuracy similar to inter-expert variability. On the EADC-ADNI dataset, we compare the hippocampal volumes obtained by manual and automatic segmentation. The volumes appear to be highly correlated that enables to perform more accurate separation of pathological populations.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge