John R. Mackey
Photon Absorption Remote Sensing Virtual Histopathology: Diagnostic Equivalence to Gold-Standard H&E Staining in Skin Cancer Excisional Biopsies
Apr 25, 2025Abstract:Photon Absorption Remote Sensing (PARS) enables label-free imaging of subcellular morphology by observing biomolecule specific absorption interactions. Coupled with deep-learning, PARS produces label-free virtual Hematoxylin and Eosin (H&E) stained images in unprocessed tissues. This study evaluates the diagnostic performance of these PARS-derived virtual H&E images in benign and malignant excisional skin biopsies, including Squamous (SCC), Basal (BCC) Cell Carcinoma, and normal skin. Sixteen unstained formalin-fixed paraffin-embedded skin excisions were PARS imaged, virtually H&E stained, then chemically stained and imaged at 40x. Seven fellowship trained dermatopathologists assessed all 32 images in a masked randomized fashion. Concordance analysis indicates 95.5% agreement between primary diagnoses rendered on PARS versus H&E images (Cohen's k=0.93). Inter-rater reliability was near-perfect for both image types (Fleiss' k=0.89 for PARS, k=0.80 for H&E). For subtype classification, agreement was near-perfect 91% (k=0.73) for SCC and was perfect for BCC. When assessing malignancy confinement (e.g., cancer margins), agreement was 92% between PARS and H&E (k=0.718). During assessment dermatopathologists could not reliably distinguish image origin (PARS vs. H&E), and diagnostic confidence was equivalent between the modalities. Inter-rater reliability for PARS virtual H&E was consistent with reported benchmarks for histologic evaluation. These results indicate that PARS virtual histology may be diagnostically equivalent to traditional H&E staining in dermatopathology diagnostics, while enabling assessment directly from unlabeled, or unprocessed slides. In turn, the label-free PARS virtual H&E imaging workflow may preserve tissue for downstream analysis while producing data well-suited for AI integration potentially accelerating and enhancing the accuracy of skin cancer diagnostics.
Automated Whole Slide Imaging for Label-Free Histology using Photon Absorption Remote Sensing Microscopy
Apr 26, 2023Abstract:The field of histology relies heavily on antiquated tissue processing and staining techniques that limit the efficiency of pathologic diagnoses of cancer and other diseases. Current staining and advanced labeling methods are often destructive and mutually incompatible, requiring new tissue sections for each stain. This prolongs the diagnostic process and depletes valuable biopsy samples. In this study, we present an alternative label-free histology platform using the first transmission-mode Photon Absorption Remote Sensing microscope. Optimized for automated whole slide scanning of unstained tissue samples, the system provides slide images at magnifications up to 40x that are fully compatible with existing digital pathology tools. The scans capture high quality and high-resolution images with subcellular diagnostic detail. After imaging, samples remain suitable for histochemical, immunohistochemical, and other staining techniques. Scattering and absorption (radiative and non-radiative) contrasts are shown in whole slide images of malignant human breast and skin tissues samples. Clinically relevant features are highlighted, and close correspondence and analogous contrast is demonstrated with one-to-one gold standard H&E stained images. Our previously reported pix2pix virtual staining model is applied to an entire whole slide image, showcasing the potential of this approach in whole slide label-free H&E emulation. This work is a critical advance for integrating label-free optical methods into standard histopathology workflows, both enhancing diagnostic efficiency, and broadening the number of stains that can be applied while preserving valuable tissue samples.
Virtual Histological Staining of Label-Free Total Absorption Photoacoustic Remote Sensing (TA-PARS)
Apr 01, 2022
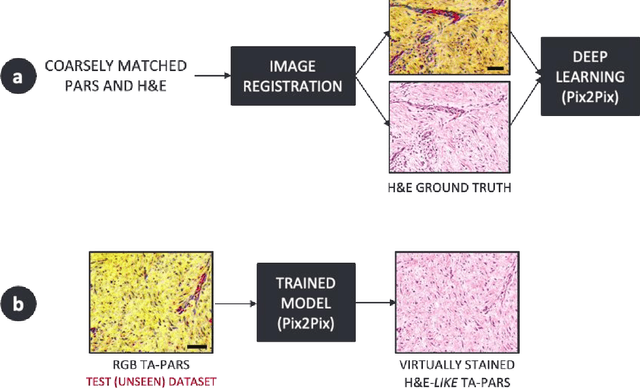

Abstract:Histopathological visualizations are a pillar of modern medicine and biological research. Surgical oncology relies exclusively on post-operative histology to determine definitive surgical success and guide adjuvant treatments. The current histology workflow is based on bright-field microscopic assessment of histochemical stained tissues and has some major limitations. For example, the preparation of stained specimens for brightfield assessment requires lengthy sample processing, delaying interventions for days or even weeks. Hence, there is a pressing need for improved histopathology methods. In this paper, we present a deep-learning-based approach for virtual label-free histochemical staining of total-absorption photoacoustic remote sensing (TA-PARS) images of unstained tissue. TA-PARS provides an array of directly measured label-free contrasts such as scattering and total absorption (radiative and non-radiative), ideal for developing H&E colorizations without the need to infer arbitrary tissue structures. We use a Pix2Pix generative adversarial network (GAN) to develop visualizations analogous to H&E staining from label-free TA-PARS images. Thin sections of human skin tissue were first virtually stained with the TA-PARS, then were chemically stained with H&E producing a one-to-one comparison between the virtual and chemical staining. The one-to-one matched virtually- and chemically- stained images exhibit high concordance validating the digital colorization of the TA-PARS images against the gold standard H&E. TA-PARS images were reviewed by four dermatologic pathologists who confirmed they are of diagnostic quality, and that resolution, contrast, and color permitted interpretation as if they were H&E. The presented approach paves the way for the development of TA-PARS slide-free histology, which promises to dramatically reduce the time from specimen resection to histological imaging.
Label-free virtual Hematoxylin and Eosin (H&E) staining using second generation Photoacoustic Remote Sensing (PARS)
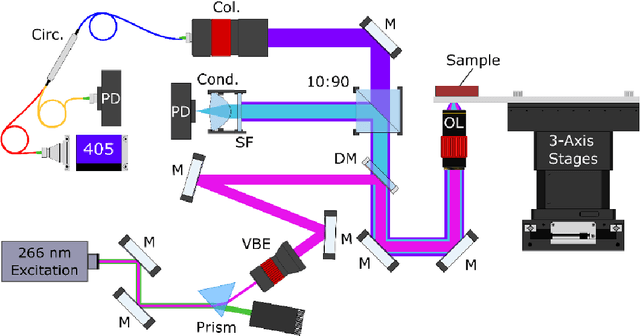
Sep 19, 2021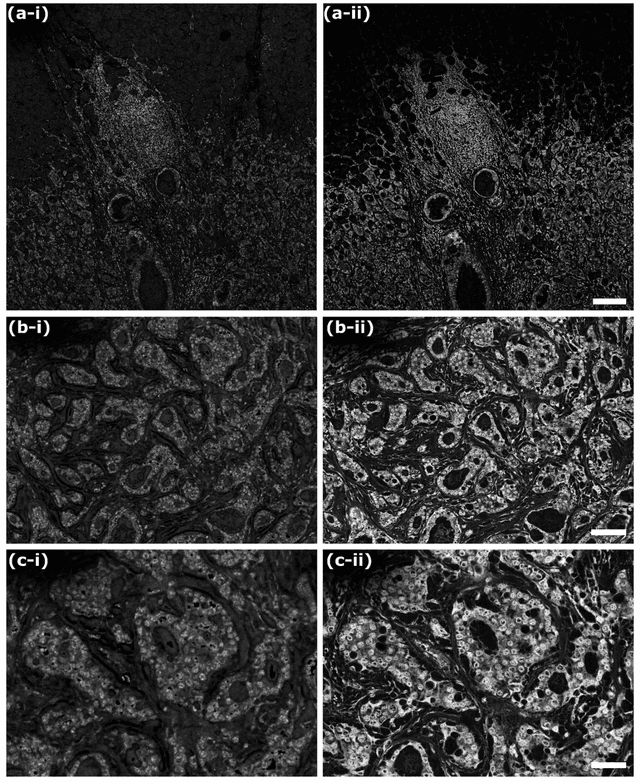
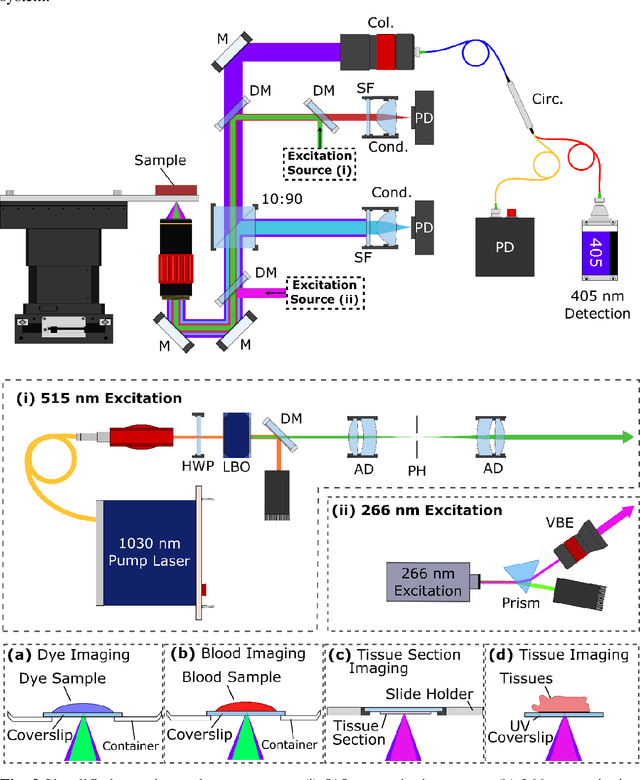
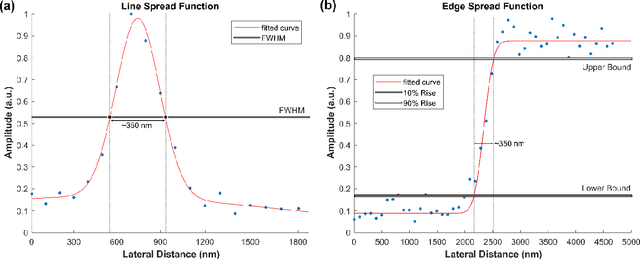
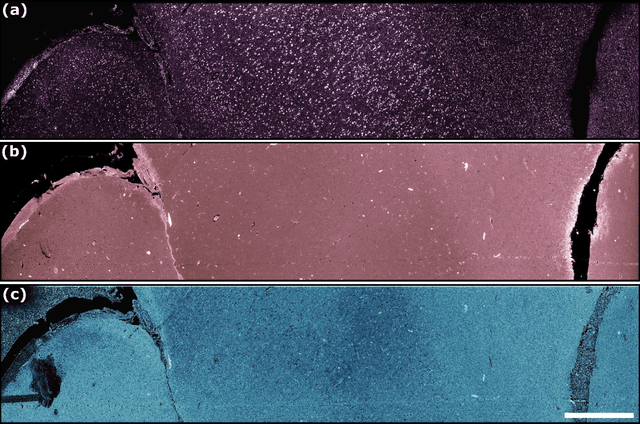
Abstract:In the past decades, absorption modalities have emerged as powerful tools for label-free functional and structural imaging of cells and tissues. Many biomolecules present unique absorption spectra providing chromophore-specific information on properties such as chemical bonding, and sample composition. As chromophores absorb photons the absorbed energy is emitted as photons (radiative relaxation) or converted to heat and under specific conditions pressure (non-radiative relaxation). Modalities like fluorescence microscopy may capture radiative relaxation to provide contrast, while modalities like photoacoustic microscopy may leverage non-radiative heat and pressures. Here we show an all-optical non-contact total-absorption photoacoustic remote sensing (TA-PARS) microscope, which can capture both radiative and non-radiative absorption effects in a single acquisition. The TA-PARS yields an absorption metric proposed as the quantum efficiency ratio (QER), which visualizes a biomolecules proportional radiative and non-radiative absorption response. The TA-PARS provides label-free visualization of a range of biomolecules enabling convincing analogues to traditional histochemical staining of tissues, effectively providing label-free Hematoxylin and Eosin (H&E)-like visualizations. These findings represent the establishment of an effective all-optical non-contact total-absorption microscope for label-free inspection of biological media.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge