Kevan Bell
Label-free virtual Hematoxylin and Eosin (H&E) staining using second generation Photoacoustic Remote Sensing (PARS)
Sep 19, 2021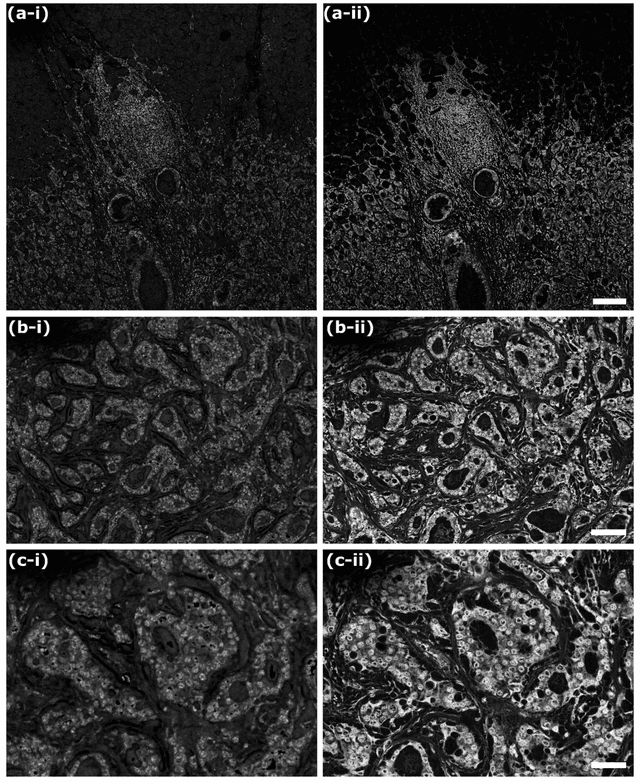
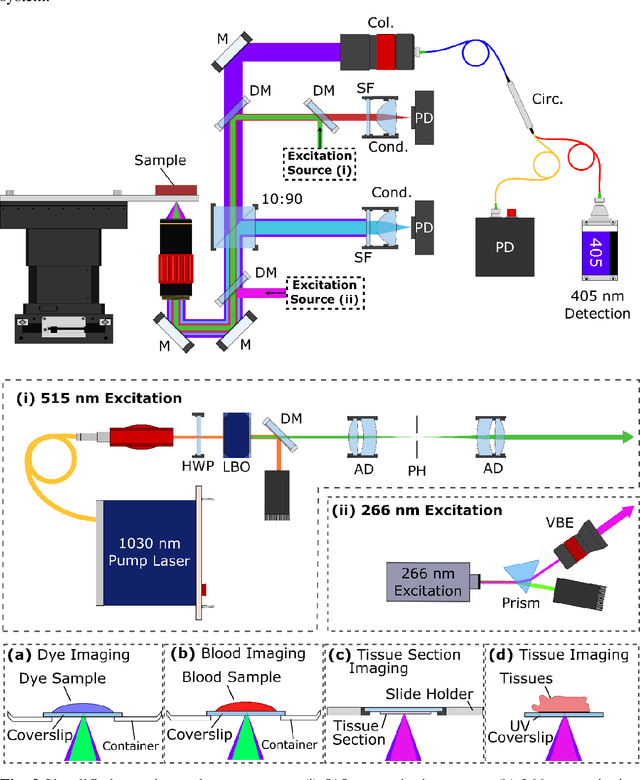
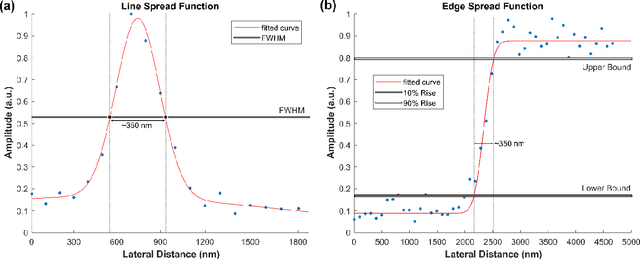
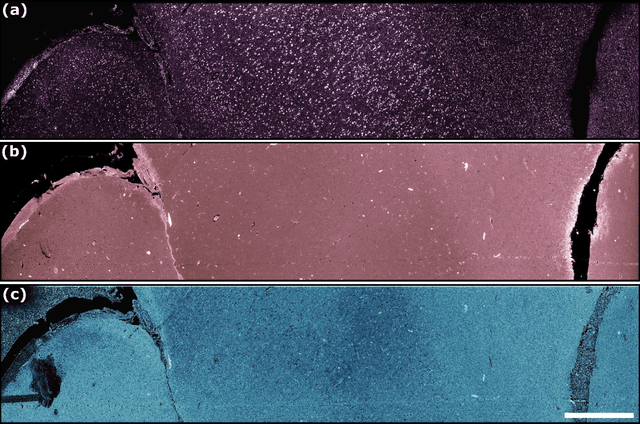
Abstract:In the past decades, absorption modalities have emerged as powerful tools for label-free functional and structural imaging of cells and tissues. Many biomolecules present unique absorption spectra providing chromophore-specific information on properties such as chemical bonding, and sample composition. As chromophores absorb photons the absorbed energy is emitted as photons (radiative relaxation) or converted to heat and under specific conditions pressure (non-radiative relaxation). Modalities like fluorescence microscopy may capture radiative relaxation to provide contrast, while modalities like photoacoustic microscopy may leverage non-radiative heat and pressures. Here we show an all-optical non-contact total-absorption photoacoustic remote sensing (TA-PARS) microscope, which can capture both radiative and non-radiative absorption effects in a single acquisition. The TA-PARS yields an absorption metric proposed as the quantum efficiency ratio (QER), which visualizes a biomolecules proportional radiative and non-radiative absorption response. The TA-PARS provides label-free visualization of a range of biomolecules enabling convincing analogues to traditional histochemical staining of tissues, effectively providing label-free Hematoxylin and Eosin (H&E)-like visualizations. These findings represent the establishment of an effective all-optical non-contact total-absorption microscope for label-free inspection of biological media.
Three-Dimensional Virtual Histology in Unprocessed Resected Tissues with Photoacoustic Remote Sensing (PARS) Microscopy and Optical Coherence Tomography
Mar 10, 2021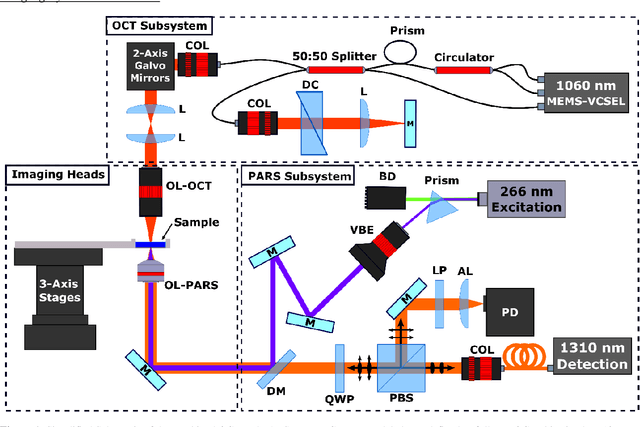
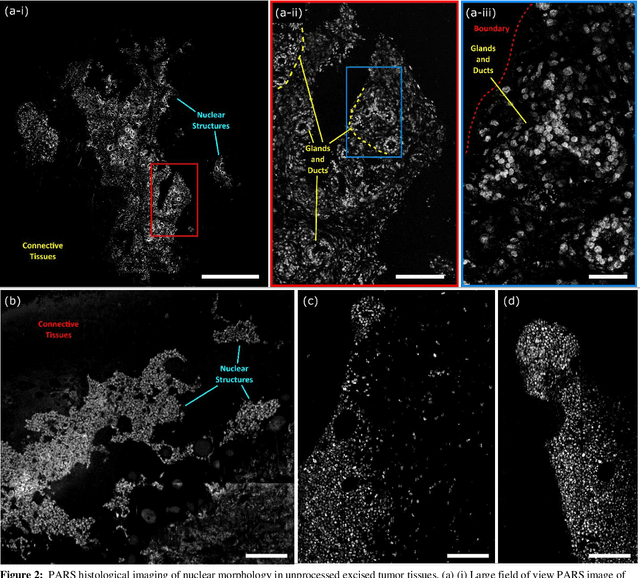

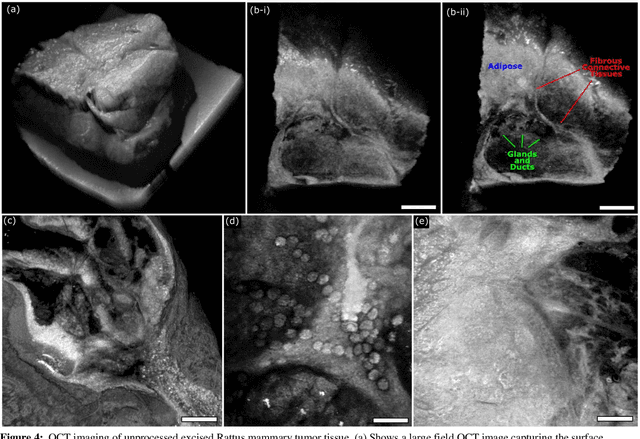
Abstract:Histological images are critical in the diagnosis and treatment of cancers. Unfortunately, the current method for capturing these microscopy images require resource intensive tissue preparation that delays diagnosis for many days to a few weeks. To streamline this process, clinicians are limited to assessing small macroscopically representative subsets of tissues. Here, we present a combined photoacoustic remote sensing (PARS) microscope and swept source optical coherence tomography (SS-OCT) system designed to circumvent these diagnostic limitations. The proposed multimodal microscope provides label-free three-dimensional depth resolved virtual histology visualizations, capturing nuclear and extranuclear tissue morphology directly on thick unprocessed specimens. The capabilities of the proposed method are demonstrated directly in unprocessed formalin fixed resected tissues. Here, we present the first images of nuclear contrast in resected human tissues, and the first 3-dimensional visualization of subsurface nuclear morphology in resected Rattus tissues, captured with a non-contact photoacoustic system. Moreover, we present the first co-registered OCT and PARS images enabling direct histological assessment of unprocessed tissues. This work represents a vital step towards the development of a real-time histological imaging modality to circumvent the limitations of current histopathology techniques.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge