Deepak Dinakaran
Photon Absorption Remote Sensing Virtual Histopathology: Diagnostic Equivalence to Gold-Standard H&E Staining in Skin Cancer Excisional Biopsies
Apr 25, 2025Abstract:Photon Absorption Remote Sensing (PARS) enables label-free imaging of subcellular morphology by observing biomolecule specific absorption interactions. Coupled with deep-learning, PARS produces label-free virtual Hematoxylin and Eosin (H&E) stained images in unprocessed tissues. This study evaluates the diagnostic performance of these PARS-derived virtual H&E images in benign and malignant excisional skin biopsies, including Squamous (SCC), Basal (BCC) Cell Carcinoma, and normal skin. Sixteen unstained formalin-fixed paraffin-embedded skin excisions were PARS imaged, virtually H&E stained, then chemically stained and imaged at 40x. Seven fellowship trained dermatopathologists assessed all 32 images in a masked randomized fashion. Concordance analysis indicates 95.5% agreement between primary diagnoses rendered on PARS versus H&E images (Cohen's k=0.93). Inter-rater reliability was near-perfect for both image types (Fleiss' k=0.89 for PARS, k=0.80 for H&E). For subtype classification, agreement was near-perfect 91% (k=0.73) for SCC and was perfect for BCC. When assessing malignancy confinement (e.g., cancer margins), agreement was 92% between PARS and H&E (k=0.718). During assessment dermatopathologists could not reliably distinguish image origin (PARS vs. H&E), and diagnostic confidence was equivalent between the modalities. Inter-rater reliability for PARS virtual H&E was consistent with reported benchmarks for histologic evaluation. These results indicate that PARS virtual histology may be diagnostically equivalent to traditional H&E staining in dermatopathology diagnostics, while enabling assessment directly from unlabeled, or unprocessed slides. In turn, the label-free PARS virtual H&E imaging workflow may preserve tissue for downstream analysis while producing data well-suited for AI integration potentially accelerating and enhancing the accuracy of skin cancer diagnostics.
Automated Whole Slide Imaging for Label-Free Histology using Photon Absorption Remote Sensing Microscopy
Apr 26, 2023Abstract:The field of histology relies heavily on antiquated tissue processing and staining techniques that limit the efficiency of pathologic diagnoses of cancer and other diseases. Current staining and advanced labeling methods are often destructive and mutually incompatible, requiring new tissue sections for each stain. This prolongs the diagnostic process and depletes valuable biopsy samples. In this study, we present an alternative label-free histology platform using the first transmission-mode Photon Absorption Remote Sensing microscope. Optimized for automated whole slide scanning of unstained tissue samples, the system provides slide images at magnifications up to 40x that are fully compatible with existing digital pathology tools. The scans capture high quality and high-resolution images with subcellular diagnostic detail. After imaging, samples remain suitable for histochemical, immunohistochemical, and other staining techniques. Scattering and absorption (radiative and non-radiative) contrasts are shown in whole slide images of malignant human breast and skin tissues samples. Clinically relevant features are highlighted, and close correspondence and analogous contrast is demonstrated with one-to-one gold standard H&E stained images. Our previously reported pix2pix virtual staining model is applied to an entire whole slide image, showcasing the potential of this approach in whole slide label-free H&E emulation. This work is a critical advance for integrating label-free optical methods into standard histopathology workflows, both enhancing diagnostic efficiency, and broadening the number of stains that can be applied while preserving valuable tissue samples.
Virtual Histological Staining of Label-Free Total Absorption Photoacoustic Remote Sensing (TA-PARS)
Apr 01, 2022
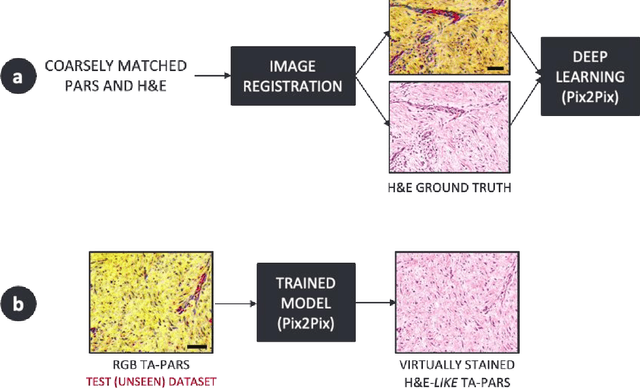

Abstract:Histopathological visualizations are a pillar of modern medicine and biological research. Surgical oncology relies exclusively on post-operative histology to determine definitive surgical success and guide adjuvant treatments. The current histology workflow is based on bright-field microscopic assessment of histochemical stained tissues and has some major limitations. For example, the preparation of stained specimens for brightfield assessment requires lengthy sample processing, delaying interventions for days or even weeks. Hence, there is a pressing need for improved histopathology methods. In this paper, we present a deep-learning-based approach for virtual label-free histochemical staining of total-absorption photoacoustic remote sensing (TA-PARS) images of unstained tissue. TA-PARS provides an array of directly measured label-free contrasts such as scattering and total absorption (radiative and non-radiative), ideal for developing H&E colorizations without the need to infer arbitrary tissue structures. We use a Pix2Pix generative adversarial network (GAN) to develop visualizations analogous to H&E staining from label-free TA-PARS images. Thin sections of human skin tissue were first virtually stained with the TA-PARS, then were chemically stained with H&E producing a one-to-one comparison between the virtual and chemical staining. The one-to-one matched virtually- and chemically- stained images exhibit high concordance validating the digital colorization of the TA-PARS images against the gold standard H&E. TA-PARS images were reviewed by four dermatologic pathologists who confirmed they are of diagnostic quality, and that resolution, contrast, and color permitted interpretation as if they were H&E. The presented approach paves the way for the development of TA-PARS slide-free histology, which promises to dramatically reduce the time from specimen resection to histological imaging.
Label-free virtual Hematoxylin and Eosin (H&E) staining using second generation Photoacoustic Remote Sensing (PARS)
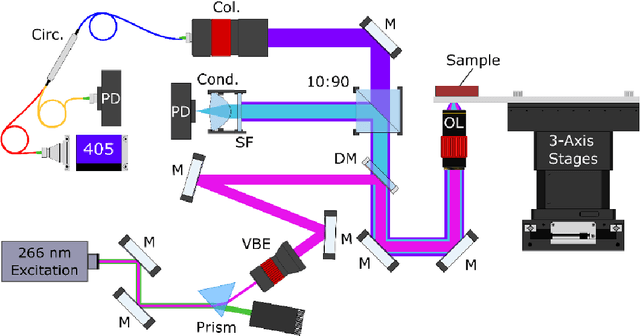
Sep 19, 2021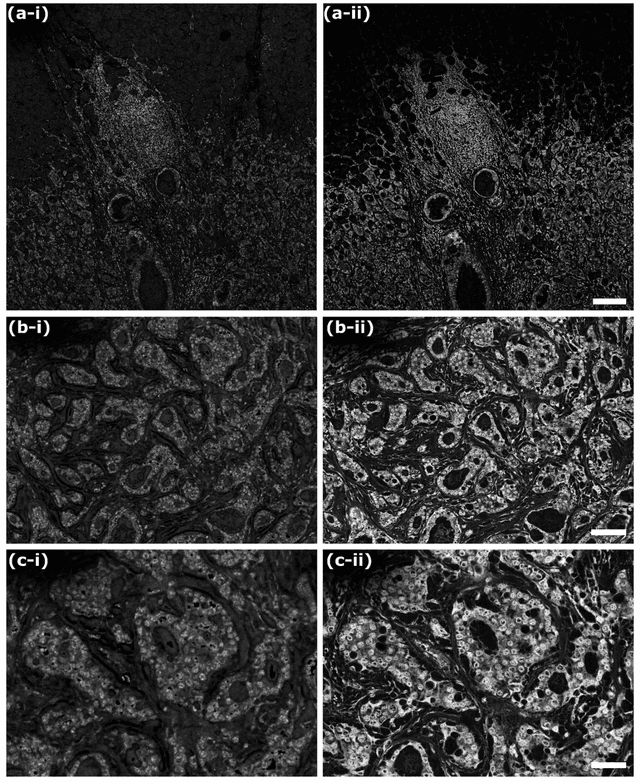
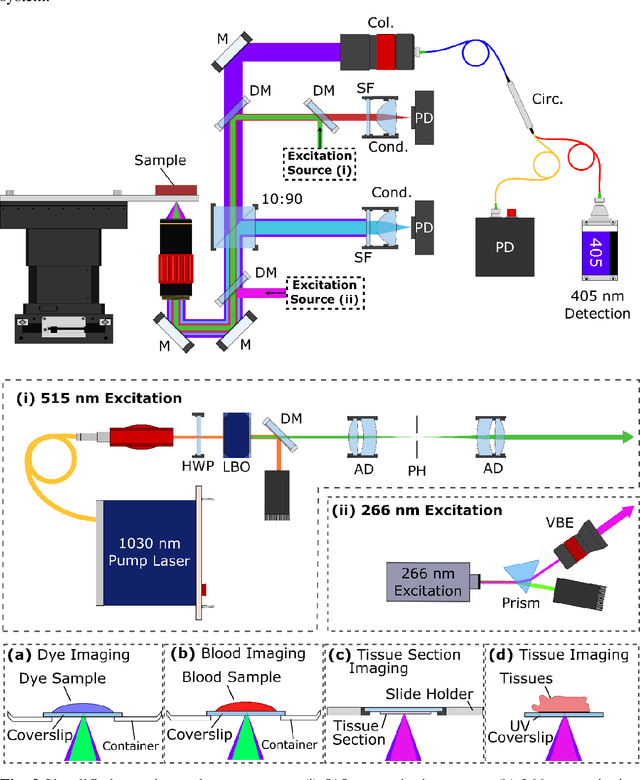
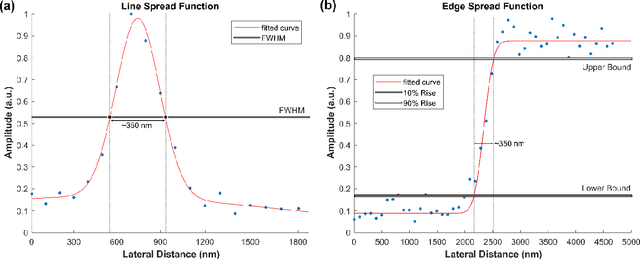
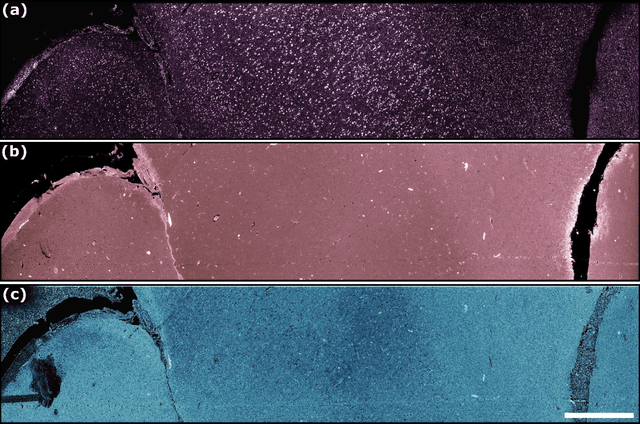
Abstract:In the past decades, absorption modalities have emerged as powerful tools for label-free functional and structural imaging of cells and tissues. Many biomolecules present unique absorption spectra providing chromophore-specific information on properties such as chemical bonding, and sample composition. As chromophores absorb photons the absorbed energy is emitted as photons (radiative relaxation) or converted to heat and under specific conditions pressure (non-radiative relaxation). Modalities like fluorescence microscopy may capture radiative relaxation to provide contrast, while modalities like photoacoustic microscopy may leverage non-radiative heat and pressures. Here we show an all-optical non-contact total-absorption photoacoustic remote sensing (TA-PARS) microscope, which can capture both radiative and non-radiative absorption effects in a single acquisition. The TA-PARS yields an absorption metric proposed as the quantum efficiency ratio (QER), which visualizes a biomolecules proportional radiative and non-radiative absorption response. The TA-PARS provides label-free visualization of a range of biomolecules enabling convincing analogues to traditional histochemical staining of tissues, effectively providing label-free Hematoxylin and Eosin (H&E)-like visualizations. These findings represent the establishment of an effective all-optical non-contact total-absorption microscope for label-free inspection of biological media.
Three-Dimensional Virtual Histology in Unprocessed Resected Tissues with Photoacoustic Remote Sensing (PARS) Microscopy and Optical Coherence Tomography
Mar 10, 2021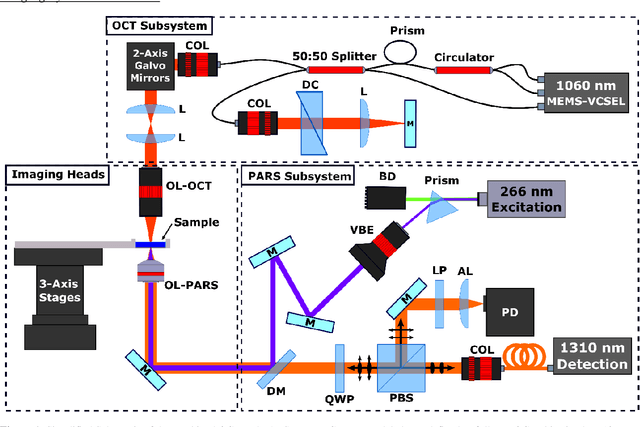
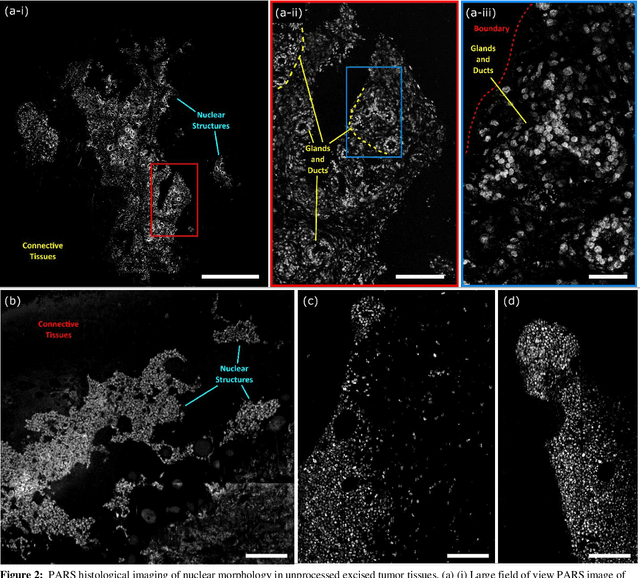

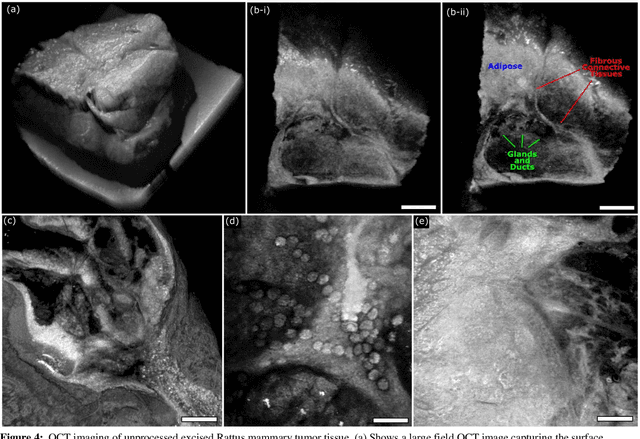
Abstract:Histological images are critical in the diagnosis and treatment of cancers. Unfortunately, the current method for capturing these microscopy images require resource intensive tissue preparation that delays diagnosis for many days to a few weeks. To streamline this process, clinicians are limited to assessing small macroscopically representative subsets of tissues. Here, we present a combined photoacoustic remote sensing (PARS) microscope and swept source optical coherence tomography (SS-OCT) system designed to circumvent these diagnostic limitations. The proposed multimodal microscope provides label-free three-dimensional depth resolved virtual histology visualizations, capturing nuclear and extranuclear tissue morphology directly on thick unprocessed specimens. The capabilities of the proposed method are demonstrated directly in unprocessed formalin fixed resected tissues. Here, we present the first images of nuclear contrast in resected human tissues, and the first 3-dimensional visualization of subsurface nuclear morphology in resected Rattus tissues, captured with a non-contact photoacoustic system. Moreover, we present the first co-registered OCT and PARS images enabling direct histological assessment of unprocessed tissues. This work represents a vital step towards the development of a real-time histological imaging modality to circumvent the limitations of current histopathology techniques.
Single Acquisition Label-free Histology-like Imaging with Dual Contrast Photoacoustic Remote Sensing Microscopy
Feb 20, 2021


Abstract:Significance: Histopathological analysis of tissues is an essential tool for grading, staging, diagnosing and resecting cancers and other malignancies. Current histopathological techniques require substantial sample processing prior to staining with hematoxylin and eosin (H&E) dyes, to highlight nuclear and cellular morphology. Sample preparation and staining is resource-intensive and introduces potential for variability during sample preparation. Aim: We present a novel method for direct label-free histopathological assessment of formalin fixed paraffin embedded tissue blocks and thin tissue sections using a dual contrast photoacoustic remote sensing (PARS) microscopy system. Approach: To emulate the nuclear and cellular contrast of H&E staining, we leverage unique properties of the PARS system. Here the ultraviolet excitation of the PAARS microscope takes advantage of DNA's unique optical absorption to provide nuclear contrast analogous to hematoxylin staining of cell nucelli. Concurrently, the optical scattering contrast of the PARS detection system is leveraged to provide bulk tissue contrast analogous to eosin staining of cell membranes. Results: We demonstrate the efficacy of this technique by imaging human breast tissue and human skin tissues in formalin fixed paraffin embedded tissue blocks and frozen sections respectively. Salient nuclear and extra-nuclear features such as cancerous cells, glands and ducts, adipocytes, and stromal structures such as collagen. Conclusions. The presented dual contrast PARS microscope enables label-free visualizations of tissue with contrast and quality analogous to the current gold standard for histopathological analysis. Thus, the proposed system is well positioned to augment existing histopathological workflows, providing histological imaging directly on unstained tissue blocks and sections.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge