Benjamin Ecclestone
Virtual Histological Staining of Label-Free Total Absorption Photoacoustic Remote Sensing (TA-PARS)
Apr 01, 2022
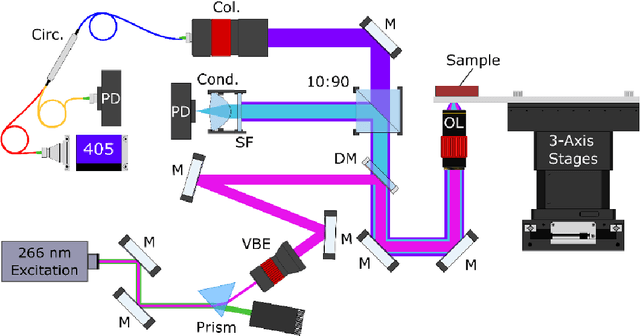
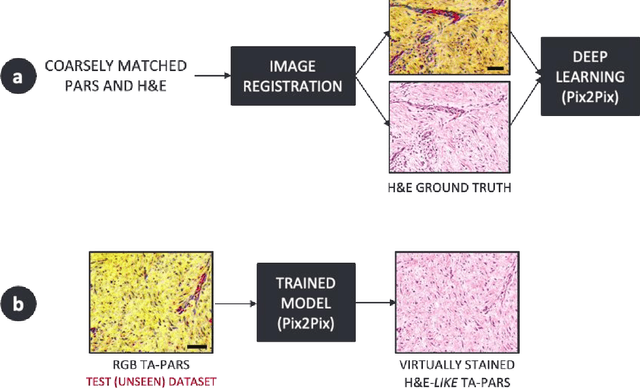

Abstract:Histopathological visualizations are a pillar of modern medicine and biological research. Surgical oncology relies exclusively on post-operative histology to determine definitive surgical success and guide adjuvant treatments. The current histology workflow is based on bright-field microscopic assessment of histochemical stained tissues and has some major limitations. For example, the preparation of stained specimens for brightfield assessment requires lengthy sample processing, delaying interventions for days or even weeks. Hence, there is a pressing need for improved histopathology methods. In this paper, we present a deep-learning-based approach for virtual label-free histochemical staining of total-absorption photoacoustic remote sensing (TA-PARS) images of unstained tissue. TA-PARS provides an array of directly measured label-free contrasts such as scattering and total absorption (radiative and non-radiative), ideal for developing H&E colorizations without the need to infer arbitrary tissue structures. We use a Pix2Pix generative adversarial network (GAN) to develop visualizations analogous to H&E staining from label-free TA-PARS images. Thin sections of human skin tissue were first virtually stained with the TA-PARS, then were chemically stained with H&E producing a one-to-one comparison between the virtual and chemical staining. The one-to-one matched virtually- and chemically- stained images exhibit high concordance validating the digital colorization of the TA-PARS images against the gold standard H&E. TA-PARS images were reviewed by four dermatologic pathologists who confirmed they are of diagnostic quality, and that resolution, contrast, and color permitted interpretation as if they were H&E. The presented approach paves the way for the development of TA-PARS slide-free histology, which promises to dramatically reduce the time from specimen resection to histological imaging.
Single Acquisition Label-free Histology-like Imaging with Dual Contrast Photoacoustic Remote Sensing Microscopy
Feb 20, 2021


Abstract:Significance: Histopathological analysis of tissues is an essential tool for grading, staging, diagnosing and resecting cancers and other malignancies. Current histopathological techniques require substantial sample processing prior to staining with hematoxylin and eosin (H&E) dyes, to highlight nuclear and cellular morphology. Sample preparation and staining is resource-intensive and introduces potential for variability during sample preparation. Aim: We present a novel method for direct label-free histopathological assessment of formalin fixed paraffin embedded tissue blocks and thin tissue sections using a dual contrast photoacoustic remote sensing (PARS) microscopy system. Approach: To emulate the nuclear and cellular contrast of H&E staining, we leverage unique properties of the PARS system. Here the ultraviolet excitation of the PAARS microscope takes advantage of DNA's unique optical absorption to provide nuclear contrast analogous to hematoxylin staining of cell nucelli. Concurrently, the optical scattering contrast of the PARS detection system is leveraged to provide bulk tissue contrast analogous to eosin staining of cell membranes. Results: We demonstrate the efficacy of this technique by imaging human breast tissue and human skin tissues in formalin fixed paraffin embedded tissue blocks and frozen sections respectively. Salient nuclear and extra-nuclear features such as cancerous cells, glands and ducts, adipocytes, and stromal structures such as collagen. Conclusions. The presented dual contrast PARS microscope enables label-free visualizations of tissue with contrast and quality analogous to the current gold standard for histopathological analysis. Thus, the proposed system is well positioned to augment existing histopathological workflows, providing histological imaging directly on unstained tissue blocks and sections.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge