Joanna Abraham
A Novel Generative Multi-Task Representation Learning Approach for Predicting Postoperative Complications in Cardiac Surgery Patients
Dec 02, 2024


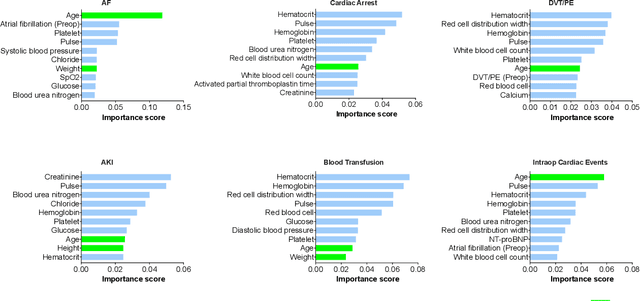
Abstract:Early detection of surgical complications allows for timely therapy and proactive risk mitigation. Machine learning (ML) can be leveraged to identify and predict patient risks for postoperative complications. We developed and validated the effectiveness of predicting postoperative complications using a novel surgical Variational Autoencoder (surgVAE) that uncovers intrinsic patterns via cross-task and cross-cohort presentation learning. This retrospective cohort study used data from the electronic health records of adult surgical patients over four years (2018 - 2021). Six key postoperative complications for cardiac surgery were assessed: acute kidney injury, atrial fibrillation, cardiac arrest, deep vein thrombosis or pulmonary embolism, blood transfusion, and other intraoperative cardiac events. We compared prediction performances of surgVAE against widely-used ML models and advanced representation learning and generative models under 5-fold cross-validation. 89,246 surgeries (49% male, median (IQR) age: 57 (45-69)) were included, with 6,502 in the targeted cardiac surgery cohort (61% male, median (IQR) age: 60 (53-70)). surgVAE demonstrated superior performance over existing ML solutions across all postoperative complications of cardiac surgery patients, achieving macro-averaged AUPRC of 0.409 and macro-averaged AUROC of 0.831, which were 3.4% and 3.7% higher, respectively, than the best alternative method (by AUPRC scores). Model interpretation using Integrated Gradients highlighted key risk factors based on preoperative variable importance. surgVAE showed excellent discriminatory performance for predicting postoperative complications and addressing the challenges of data complexity, small cohort sizes, and low-frequency positive events. surgVAE enables data-driven predictions of patient risks and prognosis while enhancing the interpretability of patient risk profiles.
Prescribing Large Language Models for Perioperative Care: What's The Right Dose for Pre-trained Models?
Feb 28, 2024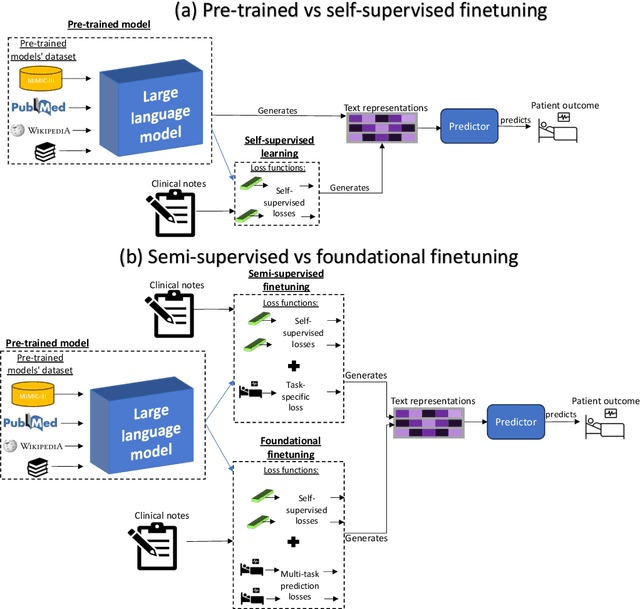



Abstract:Postoperative risk predictions can inform effective perioperative care management and planning. We aimed to assess whether clinical large language models (LLMs) can predict postoperative risks using clinical texts with various training strategies. The main cohort involved 84,875 records from Barnes Jewish Hospital (BJH) system between 2018 and 2021. Methods were replicated on Beth Israel Deaconess's MIMIC dataset. Both studies had mean duration of follow-up based on the length of postoperative ICU stay less than 7 days. For the BJH dataset, outcomes included 30-day mortality, pulmonary embolism (PE) and pneumonia. Three domain adaptation and finetuning strategies were implemented for BioGPT, ClinicalBERT and BioClinicalBERT: self-supervised objectives; incorporating labels with semi-supervised fine-tuning; and foundational modelling through multi-task learning. Model performance was compared using the area under the receiver operating characteristic curve (AUROC) and the area under the precision recall curve (AUPRC) for classification tasks, and mean squared error (MSE) and R2 for regression tasks. Pre-trained LLMs outperformed traditional word embeddings, with absolute maximal gains of 38.3% for AUROC and 14% for AUPRC. Adapting models further improved performance: (1) self-supervised finetuning by 3.2% for AUROC and 1.5% for AUPRC; (2) semi-supervised finetuning by 1.8% for AUROC and 2% for AUPRC, compared to self-supervised finetuning; (3) foundational modelling by 3.6% for AUROC and 2.6% for AUPRC, compared to self-supervised finetuning. Pre-trained clinical LLMs offer opportunities for postoperative risk predictions in unforeseen data, with peaks in foundational models indicating the potential of task-agnostic learning towards the generalizability of LLMs in perioperative care.
Self-explaining Hierarchical Model for Intraoperative Time Series
Oct 10, 2022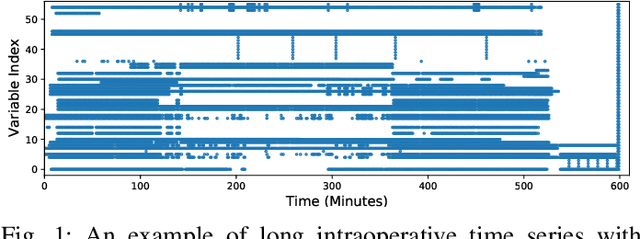
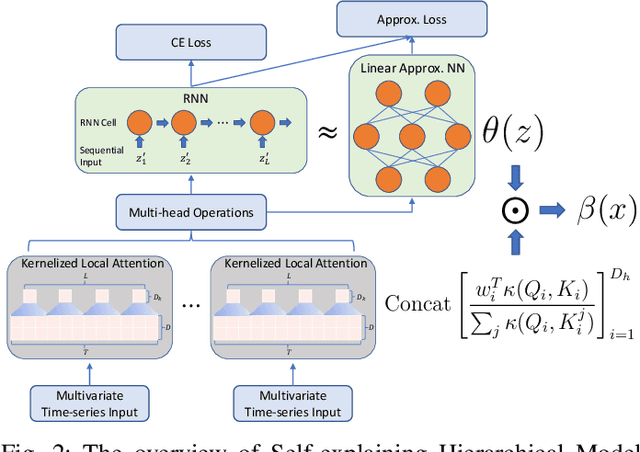
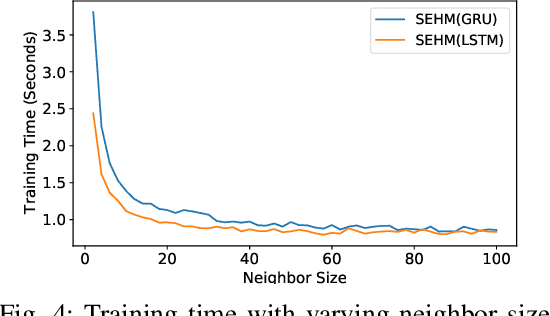
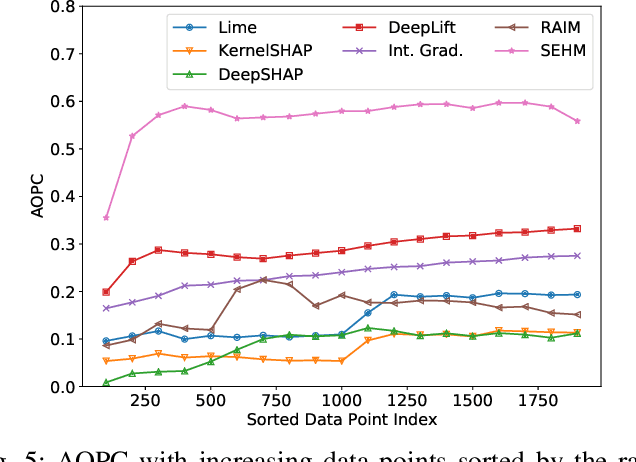
Abstract:Major postoperative complications are devastating to surgical patients. Some of these complications are potentially preventable via early predictions based on intraoperative data. However, intraoperative data comprise long and fine-grained multivariate time series, prohibiting the effective learning of accurate models. The large gaps associated with clinical events and protocols are usually ignored. Moreover, deep models generally lack transparency. Nevertheless, the interpretability is crucial to assist clinicians in planning for and delivering postoperative care and timely interventions. Towards this end, we propose a hierarchical model combining the strength of both attention and recurrent models for intraoperative time series. We further develop an explanation module for the hierarchical model to interpret the predictions by providing contributions of intraoperative data in a fine-grained manner. Experiments on a large dataset of 111,888 surgeries with multiple outcomes and an external high-resolution ICU dataset show that our model can achieve strong predictive performance (i.e., high accuracy) and offer robust interpretations (i.e., high transparency) for predicted outcomes based on intraoperative time series.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge