Junbo Shen
A Novel Generative Multi-Task Representation Learning Approach for Predicting Postoperative Complications in Cardiac Surgery Patients
Dec 02, 2024


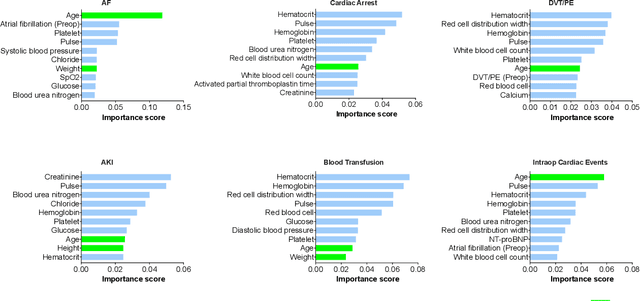
Abstract:Early detection of surgical complications allows for timely therapy and proactive risk mitigation. Machine learning (ML) can be leveraged to identify and predict patient risks for postoperative complications. We developed and validated the effectiveness of predicting postoperative complications using a novel surgical Variational Autoencoder (surgVAE) that uncovers intrinsic patterns via cross-task and cross-cohort presentation learning. This retrospective cohort study used data from the electronic health records of adult surgical patients over four years (2018 - 2021). Six key postoperative complications for cardiac surgery were assessed: acute kidney injury, atrial fibrillation, cardiac arrest, deep vein thrombosis or pulmonary embolism, blood transfusion, and other intraoperative cardiac events. We compared prediction performances of surgVAE against widely-used ML models and advanced representation learning and generative models under 5-fold cross-validation. 89,246 surgeries (49% male, median (IQR) age: 57 (45-69)) were included, with 6,502 in the targeted cardiac surgery cohort (61% male, median (IQR) age: 60 (53-70)). surgVAE demonstrated superior performance over existing ML solutions across all postoperative complications of cardiac surgery patients, achieving macro-averaged AUPRC of 0.409 and macro-averaged AUROC of 0.831, which were 3.4% and 3.7% higher, respectively, than the best alternative method (by AUPRC scores). Model interpretation using Integrated Gradients highlighted key risk factors based on preoperative variable importance. surgVAE showed excellent discriminatory performance for predicting postoperative complications and addressing the challenges of data complexity, small cohort sizes, and low-frequency positive events. surgVAE enables data-driven predictions of patient risks and prognosis while enhancing the interpretability of patient risk profiles.
Unbiased organism-agnostic and highly sensitive signal peptide predictor with deep protein language model
Dec 14, 2023Abstract:Signal peptide (SP) is a short peptide located in the N-terminus of proteins. It is essential to target and transfer transmembrane and secreted proteins to correct positions. Compared with traditional experimental methods to identify signal peptides, computational methods are faster and more efficient, which are more practical for analyzing thousands or even millions of protein sequences, especially for metagenomic data. Here we present Unbiased Organism-agnostic Signal Peptide Network (USPNet), a signal peptide classification and cleavage site prediction deep learning method that takes advantage of protein language models. We propose to apply label distribution-aware margin loss to handle data imbalance problems and use evolutionary information of protein to enrich representation and overcome species information dependence.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge