Jiyang Jiang
Computer-Aided Extraction of Select MRI Markers of Cerebral Small Vessel Disease: A Systematic Review
Apr 04, 2022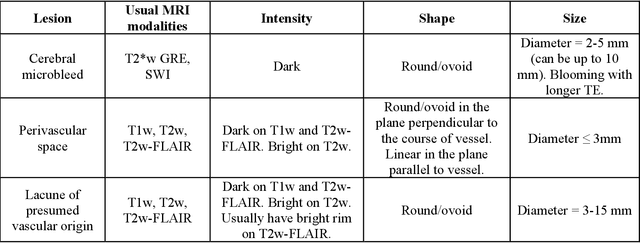
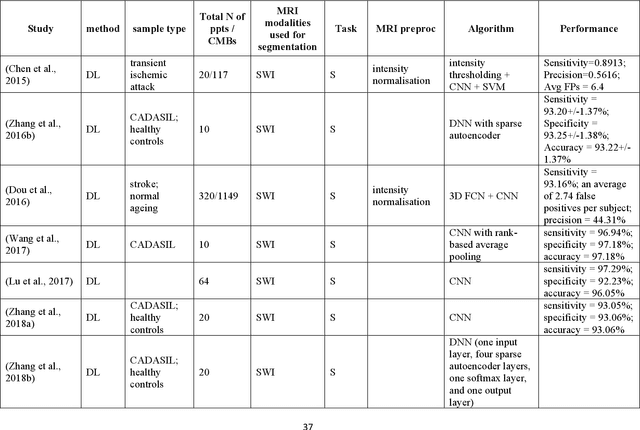
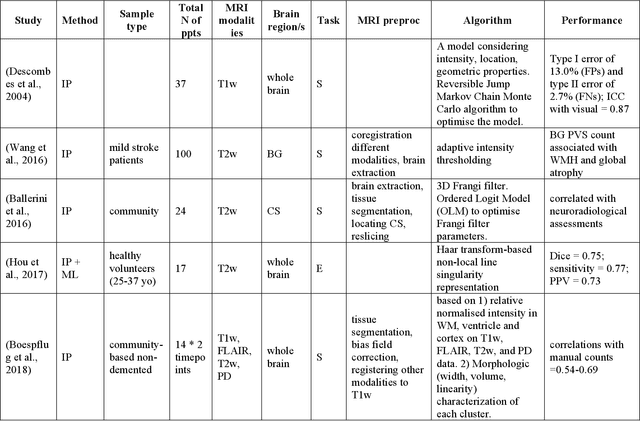
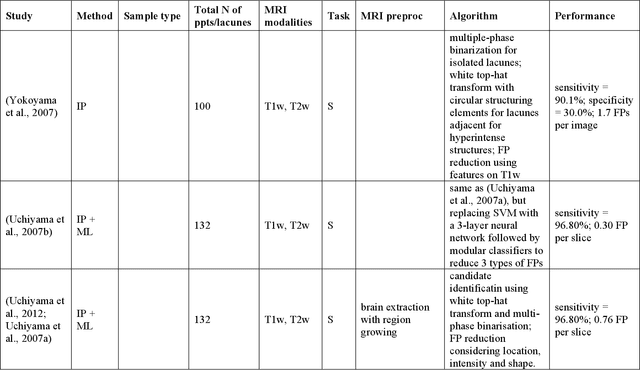
Abstract:Cerebral small vessel disease (CSVD) is a major vascular contributor to cognitive impairment in ageing, including dementias. Imaging remains the most promising method for in vivo studies of CSVD. To replace the subjective and laborious visual rating approaches, emerging studies have applied state-of-the-art artificial intelligence to extract imaging biomarkers of CSVD from MRI scans. We aimed to summarise published computer-aided methods to examine three imaging biomarkers of CSVD, namely cerebral microbleeds (CMB), dilated perivascular spaces (PVS), and lacunes of presumed vascular origin. Seventy-one classical image processing, classical machine learning, and deep learning studies were identified. CMB and PVS have been better studied, compared to lacunes. While good performance metrics have been achieved in local test datasets, there have not been generalisable pipelines validated in different research or clinical cohorts. Transfer learning and weak supervision techniques have been applied to accommodate the limitations in training data. Future studies could consider pooling data from multiple sources to increase diversity, and validating the performance of the methods using both image processing metrics and associations with clinical measures.
Brain Age Estimation From MRI Using Cascade Networks with Ranking Loss
Jun 06, 2021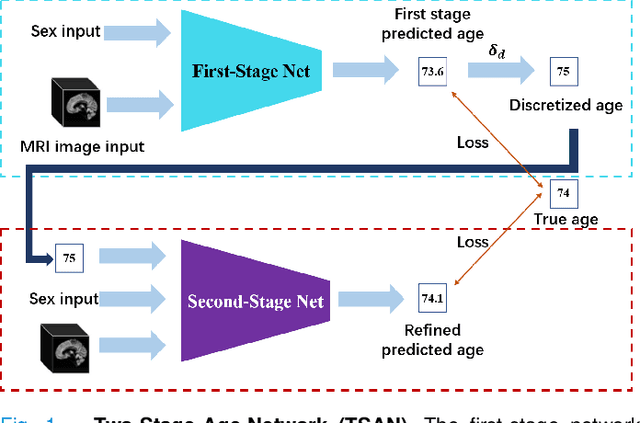
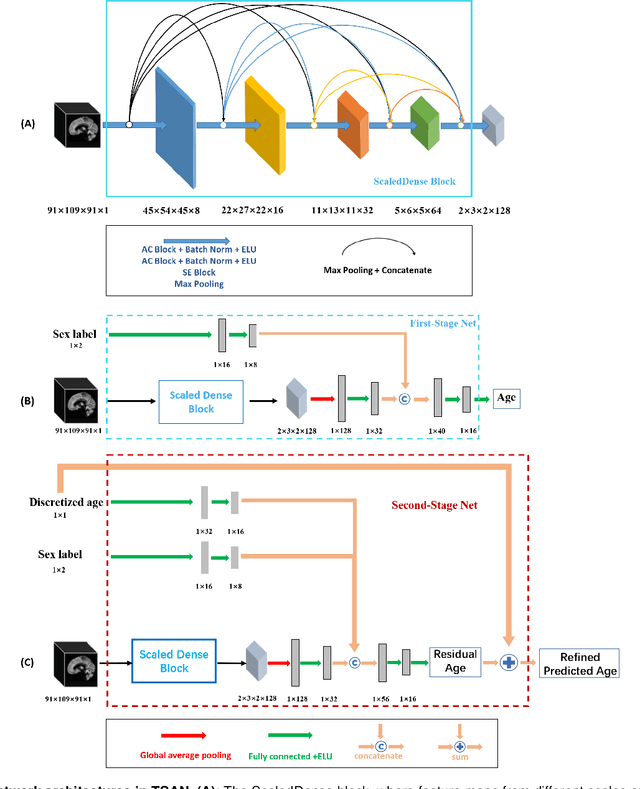
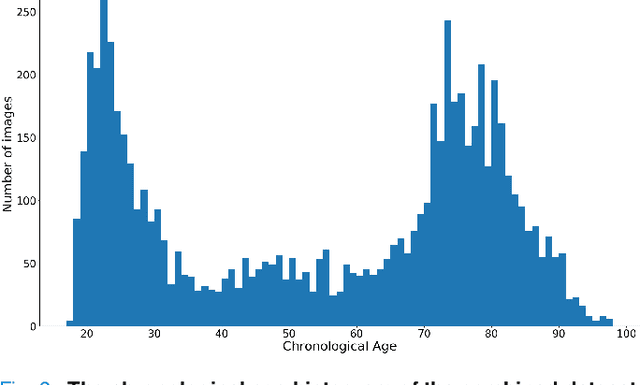
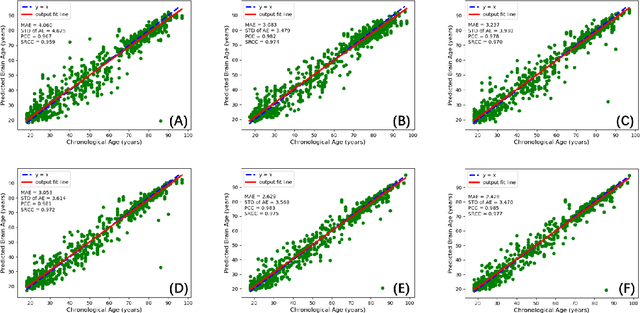
Abstract:Chronological age of healthy people is able to be predicted accurately using deep neural networks from neuroimaging data, and the predicted brain age could serve as a biomarker for detecting aging-related diseases. In this paper, a novel 3D convolutional network, called two-stage-age-network (TSAN), is proposed to estimate brain age from T1-weighted MRI data. Compared with existing methods, TSAN has the following improvements. First, TSAN uses a two-stage cascade network architecture, where the first-stage network estimates a rough brain age, then the second-stage network estimates the brain age more accurately from the discretized brain age by the first-stage network. Second, to our knowledge, TSAN is the first work to apply novel ranking losses in brain age estimation, together with the traditional mean square error (MSE) loss. Third, densely connected paths are used to combine feature maps with different scales. The experiments with $6586$ MRIs showed that TSAN could provide accurate brain age estimation, yielding mean absolute error (MAE) of $2.428$ and Pearson's correlation coefficient (PCC) of $0.985$, between the estimated and chronological ages. Furthermore, using the brain age gap between brain age and chronological age as a biomarker, Alzheimer's disease (AD) and Mild Cognitive Impairment (MCI) can be distinguished from healthy control (HC) subjects by support vector machine (SVM). Classification AUC in AD/HC and MCI/HC was $0.904$ and $0.823$, respectively. It showed that brain age gap is an effective biomarker associated with risk of dementia, and has potential for early-stage dementia risk screening. The codes and trained models have been released on GitHub: https://github.com/Milan-BUAA/TSAN-brain-age-estimation.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge