Jeremias Traub
Finally Outshining the Random Baseline: A Simple and Effective Solution for Active Learning in 3D Biomedical Imaging
Jan 20, 2026Abstract:Active learning (AL) has the potential to drastically reduce annotation costs in 3D biomedical image segmentation, where expert labeling of volumetric data is both time-consuming and expensive. Yet, existing AL methods are unable to consistently outperform improved random sampling baselines adapted to 3D data, leaving the field without a reliable solution. We introduce Class-stratified Scheduled Power Predictive Entropy (ClaSP PE), a simple and effective query strategy that addresses two key limitations of standard uncertainty-based AL methods: class imbalance and redundancy in early selections. ClaSP PE combines class-stratified querying to ensure coverage of underrepresented structures and log-scale power noising with a decaying schedule to enforce query diversity in early-stage AL and encourage exploitation later. In our evaluation on 24 experimental settings using four 3D biomedical datasets within the comprehensive nnActive benchmark, ClaSP PE is the only method that generally outperforms improved random baselines in terms of both segmentation quality with statistically significant gains, whilst remaining annotation efficient. Furthermore, we explicitly simulate the real-world application by testing our method on four previously unseen datasets without manual adaptation, where all experiment parameters are set according to predefined guidelines. The results confirm that ClaSP PE robustly generalizes to novel tasks without requiring dataset-specific tuning. Within the nnActive framework, we present compelling evidence that an AL method can consistently outperform random baselines adapted to 3D segmentation, in terms of both performance and annotation efficiency in a realistic, close-to-production scenario. Our open-source implementation and clear deployment guidelines make it readily applicable in practice. Code is at https://github.com/MIC-DKFZ/nnActive.
SURE-VQA: Systematic Understanding of Robustness Evaluation in Medical VQA Tasks
Nov 29, 2024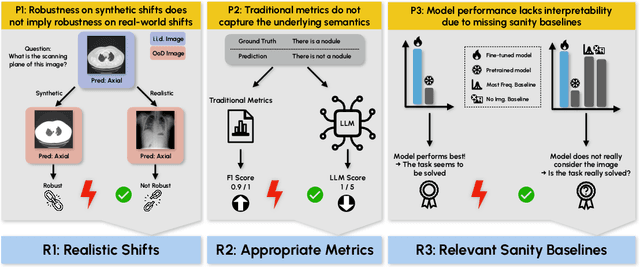
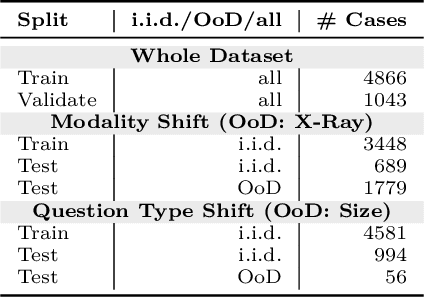
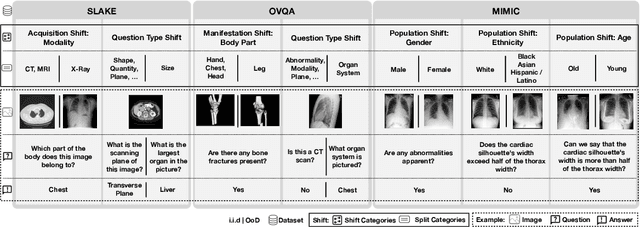
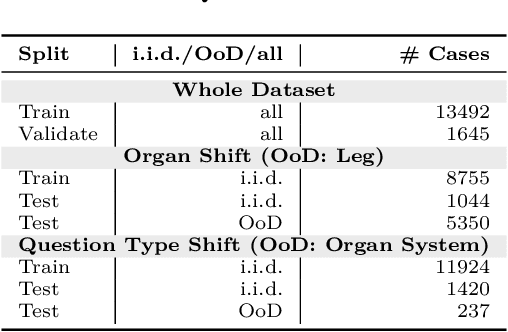
Abstract:Vision-Language Models (VLMs) have great potential in medical tasks, like Visual Question Answering (VQA), where they could act as interactive assistants for both patients and clinicians. Yet their robustness to distribution shifts on unseen data remains a critical concern for safe deployment. Evaluating such robustness requires a controlled experimental setup that allows for systematic insights into the model's behavior. However, we demonstrate that current setups fail to offer sufficiently thorough evaluations, limiting their ability to accurately assess model robustness. To address this gap, our work introduces a novel framework, called SURE-VQA, centered around three key requirements to overcome the current pitfalls and systematically analyze the robustness of VLMs: 1) Since robustness on synthetic shifts does not necessarily translate to real-world shifts, robustness should be measured on real-world shifts that are inherent to the VQA data; 2) Traditional token-matching metrics often fail to capture underlying semantics, necessitating the use of large language models (LLMs) for more accurate semantic evaluation; 3) Model performance often lacks interpretability due to missing sanity baselines, thus meaningful baselines should be reported that allow assessing the multimodal impact on the VLM. To demonstrate the relevance of this framework, we conduct a study on the robustness of various fine-tuning methods across three medical datasets with four different types of distribution shifts. Our study reveals several important findings: 1) Sanity baselines that do not utilize image data can perform surprisingly well; 2) We confirm LoRA as the best-performing PEFT method; 3) No PEFT method consistently outperforms others in terms of robustness to shifts. Code is provided at https://github.com/IML-DKFZ/sure-vqa.
Overcoming Common Flaws in the Evaluation of Selective Classification Systems
Jul 01, 2024



Abstract:Selective Classification, wherein models can reject low-confidence predictions, promises reliable translation of machine-learning based classification systems to real-world scenarios such as clinical diagnostics. While current evaluation of these systems typically assumes fixed working points based on pre-defined rejection thresholds, methodological progress requires benchmarking the general performance of systems akin to the $\mathrm{AUROC}$ in standard classification. In this work, we define 5 requirements for multi-threshold metrics in selective classification regarding task alignment, interpretability, and flexibility, and show how current approaches fail to meet them. We propose the Area under the Generalized Risk Coverage curve ($\mathrm{AUGRC}$), which meets all requirements and can be directly interpreted as the average risk of undetected failures. We empirically demonstrate the relevance of $\mathrm{AUGRC}$ on a comprehensive benchmark spanning 6 data sets and 13 confidence scoring functions. We find that the proposed metric substantially changes metric rankings on 5 out of the 6 data sets.
Comparative Benchmarking of Failure Detection Methods in Medical Image Segmentation: Unveiling the Role of Confidence Aggregation
Jun 05, 2024



Abstract:Semantic segmentation is an essential component of medical image analysis research, with recent deep learning algorithms offering out-of-the-box applicability across diverse datasets. Despite these advancements, segmentation failures remain a significant concern for real-world clinical applications, necessitating reliable detection mechanisms. This paper introduces a comprehensive benchmarking framework aimed at evaluating failure detection methodologies within medical image segmentation. Through our analysis, we identify the strengths and limitations of current failure detection metrics, advocating for the risk-coverage analysis as a holistic evaluation approach. Utilizing a collective dataset comprising five public 3D medical image collections, we assess the efficacy of various failure detection strategies under realistic test-time distribution shifts. Our findings highlight the importance of pixel confidence aggregation and we observe superior performance of the pairwise Dice score (Roy et al., 2019) between ensemble predictions, positioning it as a simple and robust baseline for failure detection in medical image segmentation. To promote ongoing research, we make the benchmarking framework available to the community.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge