Jean-Paul Charbonnier
Emphysema Subtyping on Thoracic Computed Tomography Scans using Deep Neural Networks
Sep 05, 2023Abstract:Accurate identification of emphysema subtypes and severity is crucial for effective management of COPD and the study of disease heterogeneity. Manual analysis of emphysema subtypes and severity is laborious and subjective. To address this challenge, we present a deep learning-based approach for automating the Fleischner Society's visual score system for emphysema subtyping and severity analysis. We trained and evaluated our algorithm using 9650 subjects from the COPDGene study. Our algorithm achieved the predictive accuracy at 52\%, outperforming a previously published method's accuracy of 45\%. In addition, the agreement between the predicted scores of our method and the visual scores was good, where the previous method obtained only moderate agreement. Our approach employs a regression training strategy to generate categorical labels while simultaneously producing high-resolution localized activation maps for visualizing the network predictions. By leveraging these dense activation maps, our method possesses the capability to compute the percentage of emphysema involvement per lung in addition to categorical severity scores. Furthermore, the proposed method extends its predictive capabilities beyond centrilobular emphysema to include paraseptal emphysema subtypes.
Data Harmonisation for Information Fusion in Digital Healthcare: A State-of-the-Art Systematic Review, Meta-Analysis and Future Research Directions
Jan 17, 2022

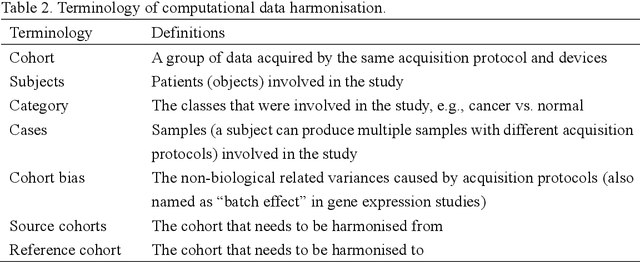

Abstract:Removing the bias and variance of multicentre data has always been a challenge in large scale digital healthcare studies, which requires the ability to integrate clinical features extracted from data acquired by different scanners and protocols to improve stability and robustness. Previous studies have described various computational approaches to fuse single modality multicentre datasets. However, these surveys rarely focused on evaluation metrics and lacked a checklist for computational data harmonisation studies. In this systematic review, we summarise the computational data harmonisation approaches for multi-modality data in the digital healthcare field, including harmonisation strategies and evaluation metrics based on different theories. In addition, a comprehensive checklist that summarises common practices for data harmonisation studies is proposed to guide researchers to report their research findings more effectively. Last but not least, flowcharts presenting possible ways for methodology and metric selection are proposed and the limitations of different methods have been surveyed for future research.
Structure and position-aware graph neural network for airway labeling
Jan 12, 2022



Abstract:We present a novel graph-based approach for labeling the anatomical branches of a given airway tree segmentation. The proposed method formulates airway labeling as a branch classification problem in the airway tree graph, where branch features are extracted using convolutional neural networks (CNN) and enriched using graph neural networks. Our graph neural network is structure-aware by having each node aggregate information from its local neighbors and position-aware by encoding node positions in the graph. We evaluated the proposed method on 220 airway trees from subjects with various severity stages of Chronic Obstructive Pulmonary Disease (COPD). The results demonstrate that our approach is computationally efficient and significantly improves branch classification performance than the baseline method. The overall average accuracy of our method reaches 91.18\% for labeling all 18 segmental airway branches, compared to 83.83\% obtained by the standard CNN method. We published our source code at https://github.com/DIAGNijmegen/spgnn. The proposed algorithm is also publicly available at https://grand-challenge.org/algorithms/airway-anatomical-labeling/.
Relational Modeling for Robust and Efficient Pulmonary Lobe Segmentation in CT Scans
May 12, 2020



Abstract:Pulmonary lobe segmentation in computed tomography scans is essential for regional assessment of pulmonary diseases. Recent works based on convolution neural networks have achieved good performance for this task. However, they are still limited in capturing structured relationships due to the nature of convolution. The shape of the pulmonary lobes affect each other and their borders relate to the appearance of other structures, such as vessels, airways, and the pleural wall. We argue that such structural relationships play a critical role in the accurate delineation of pulmonary lobes when the lungs are affected by diseases such as COVID-19 or COPD. In this paper, we propose a relational approach (RTSU-Net) that leverages structured relationships by introducing a novel non-local neural network module. The proposed module learns both visual and geometric relationships among all convolution features to produce self-attention weights. With a limited amount of training data available from COVID-19 subjects, we initially train and validate RTSU-Net on a cohort of 5000 subjects from the COPDGene study (4000 for training and 1000 for evaluation). Using models pre-trained on COPDGene, we apply transfer learning to retrain and evaluate RTSU-Net on 470 COVID-19 suspects (370 for retraining and 100 for evaluation). Experimental results show that RTSU-Net outperforms three baselines and performs robustly on cases with severe lung infection due to COVID-19.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge