Jay J. Pillai
Deep-learning-enabled Brain Hemodynamic Mapping Using Resting-state fMRI
Apr 25, 2022



Abstract:Cerebrovascular disease is a leading cause of death globally. Prevention and early intervention are known to be the most effective forms of its management. Non-invasive imaging methods hold great promises for early stratification, but at present lack the sensitivity for personalized prognosis. Resting-state functional magnetic resonance imaging (rs-fMRI), a powerful tool previously used for mapping neural activity, is available in most hospitals. Here we show that rs-fMRI can be used to map cerebral hemodynamic function and delineate impairment. By exploiting time variations in breathing pattern during rs-fMRI, deep learning enables reproducible mapping of cerebrovascular reactivity (CVR) and bolus arrive time (BAT) of the human brain using resting-state CO2 fluctuations as a natural 'contrast media'. The deep-learning network was trained with CVR and BAT maps obtained with a reference method of CO2-inhalation MRI, which included data from young and older healthy subjects and patients with Moyamoya disease and brain tumors. We demonstrate the performance of deep-learning cerebrovascular mapping in the detection of vascular abnormalities, evaluation of revascularization effects, and vascular alterations in normal aging. In addition, cerebrovascular maps obtained with the proposed method exhibited excellent reproducibility in both healthy volunteers and stroke patients. Deep-learning resting-state vascular imaging has the potential to become a useful tool in clinical cerebrovascular imaging.
A Multi-Task Deep Learning Framework to Localize the Eloquent Cortex in Brain Tumor Patients Using Dynamic Functional Connectivity
Nov 17, 2020



Abstract:We present a novel deep learning framework that uses dynamic functional connectivity to simultaneously localize the language and motor areas of the eloquent cortex in brain tumor patients. Our method leverages convolutional layers to extract graph-based features from the dynamic connectivity matrices and a long-short term memory (LSTM) attention network to weight the relevant time points during classification. The final stage of our model employs multi-task learning to identify different eloquent subsystems. Our unique training strategy finds a shared representation between the cognitive networks of interest, which enables us to handle missing patient data. We evaluate our method on resting-state fMRI data from 56 brain tumor patients while using task fMRI activations as surrogate ground-truth labels for training and testing. Our model achieves higher localization accuracies than conventional deep learning approaches and can identify bilateral language areas even when trained on left-hemisphere lateralized cases. Hence, our method may ultimately be useful for preoperative mapping in tumor patients.
Multiparametric Deep Learning and Radiomics for Tumor Grading and Treatment Response Assessment of Brain Cancer: Preliminary Results
Jun 10, 2019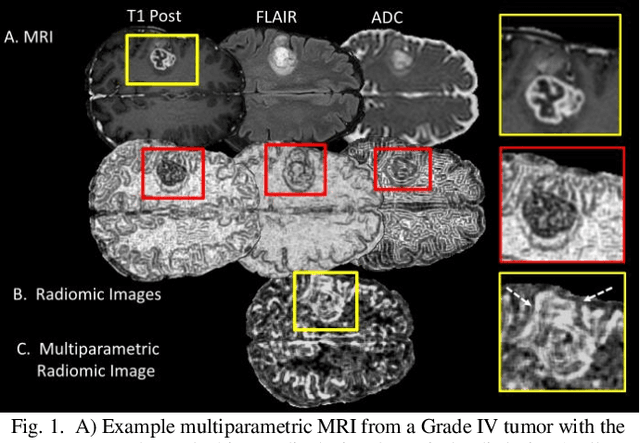
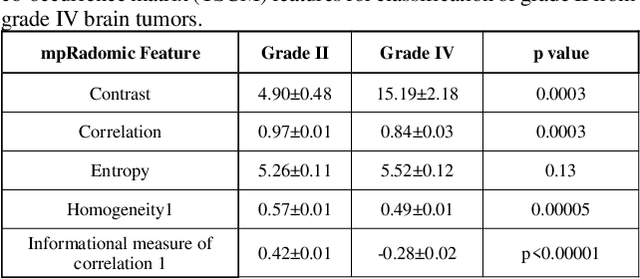
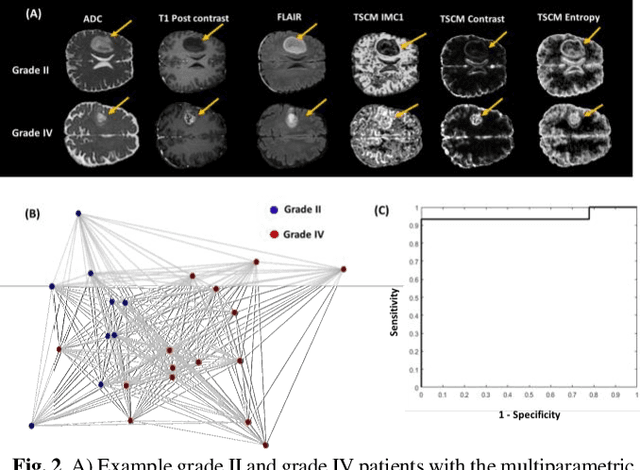
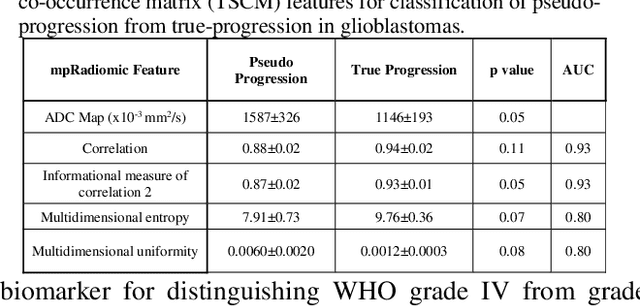
Abstract:Radiomics is an exciting new area of texture research for extracting quantitative and morphological characteristics of pathological tissue. However, to date, only single images have been used for texture analysis. We have extended radiomic texture methods to use multiparametric (mp) data to get more complete information from all the images. These mpRadiomic methods could potentially provide a platform for stratification of tumor grade as well as assessment of treatment response in brain tumors. In brain, multiparametric MRI (mpMRI) are based on contrast enhanced T1-weighted imaging (T1WI), T2WI, Fluid Attenuated Inversion Recovery (FLAIR), Diffusion Weighted Imaging (DWI) and Perfusion Weighted Imaging (PWI). Therefore, we applied our multiparametric radiomic framework (mpRadiomic) on 24 patients with brain tumors (8 grade II and 16 grade IV). The mpRadiomic framework classified grade IV tumors from grade II tumors with a sensitivity and specificity of 93% and 100%, respectively, with an AUC of 0.95. For treatment response, the mpRadiomic framework classified pseudo-progression from true-progression with an AUC of 0.93. In conclusion, the mpRadiomic analysis was able to effectively capture the multiparametric brain MRI texture and could be used as potential biomarkers for distinguishing grade IV from grade II tumors as well as determining true-progression from pseudo-progression.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge