Janani Iyer
Self-training of Machine Learning Models for Liver Histopathology: Generalization under Clinical Shifts
Nov 14, 2022

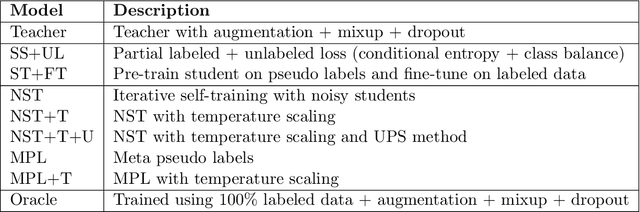

Abstract:Histopathology images are gigapixel-sized and include features and information at different resolutions. Collecting annotations in histopathology requires highly specialized pathologists, making it expensive and time-consuming. Self-training can alleviate annotation constraints by learning from both labeled and unlabeled data, reducing the amount of annotations required from pathologists. We study the design of teacher-student self-training systems for Non-alcoholic Steatohepatitis (NASH) using clinical histopathology datasets with limited annotations. We evaluate the models on in-distribution and out-of-distribution test data under clinical data shifts. We demonstrate that through self-training, the best student model statistically outperforms the teacher with a $3\%$ absolute difference on the macro F1 score. The best student model also approaches the performance of a fully supervised model trained with twice as many annotations.
Multi stain graph fusion for multimodal integration in pathology
Apr 26, 2022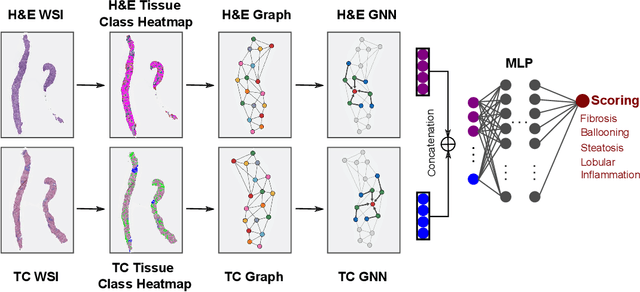
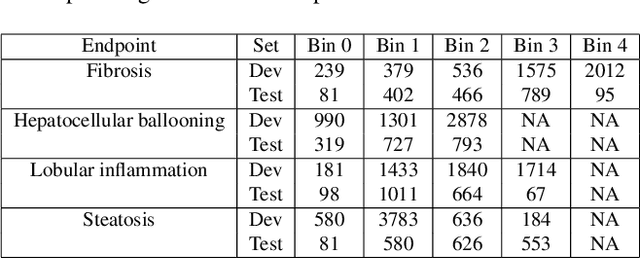
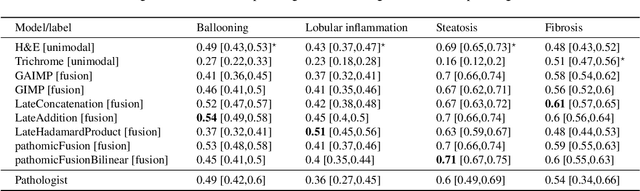
Abstract:In pathology, tissue samples are assessed using multiple staining techniques to enhance contrast in unique histologic features. In this paper, we introduce a multimodal CNN-GNN based graph fusion approach that leverages complementary information from multiple non-registered histopathology images to predict pathologic scores. We demonstrate this approach in nonalcoholic steatohepatitis (NASH) by predicting CRN fibrosis stage and NAFLD Activity Score (NAS). Primary assessment of NASH typically requires liver biopsy evaluation on two histological stains: Trichrome (TC) and hematoxylin and eosin (H&E). Our multimodal approach learns to extract complementary information from TC and H&E graphs corresponding to each stain while simultaneously learning an optimal policy to combine this information. We report up to 20% improvement in predicting fibrosis stage and NAS component grades over single-stain modeling approaches, measured by computing linearly weighted Cohen's kappa between machine-derived vs. pathologist consensus scores. Broadly, this paper demonstrates the value of leveraging diverse pathology images for improved ML-powered histologic assessment.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge