Ioannis Pappas
What makes a face looks like a hat: Decoupling low-level and high-level Visual Properties with Image Triplets
Sep 03, 2024



Abstract:In visual decision making, high-level features, such as object categories, have a strong influence on choice. However, the impact of low-level features on behavior is less understood partly due to the high correlation between high- and low-level features in the stimuli presented (e.g., objects of the same category are more likely to share low-level features). To disentangle these effects, we propose a method that de-correlates low- and high-level visual properties in a novel set of stimuli. Our method uses two Convolutional Neural Networks (CNNs) as candidate models of the ventral visual stream: the CORnet-S that has high neural predictivity in high-level, IT-like responses and the VGG-16 that has high neural predictivity in low-level responses. Triplets (root, image1, image2) of stimuli are parametrized by the level of low- and high-level similarity of images extracted from the different layers. These stimuli are then used in a decision-making task where participants are tasked to choose the most similar-to-the-root image. We found that different networks show differing abilities to predict the effects of low-versus-high-level similarity: while CORnet-S outperforms VGG-16 in explaining human choices based on high-level similarity, VGG-16 outperforms CORnet-S in explaining human choices based on low-level similarity. Using Brain-Score, we observed that the behavioral prediction abilities of different layers of these networks qualitatively corresponded to their ability to explain neural activity at different levels of the visual hierarchy. In summary, our algorithm for stimulus set generation enables the study of how different representations in the visual stream affect high-level cognitive behaviors.
A Robust Ensemble Algorithm for Ischemic Stroke Lesion Segmentation: Generalizability and Clinical Utility Beyond the ISLES Challenge
Apr 03, 2024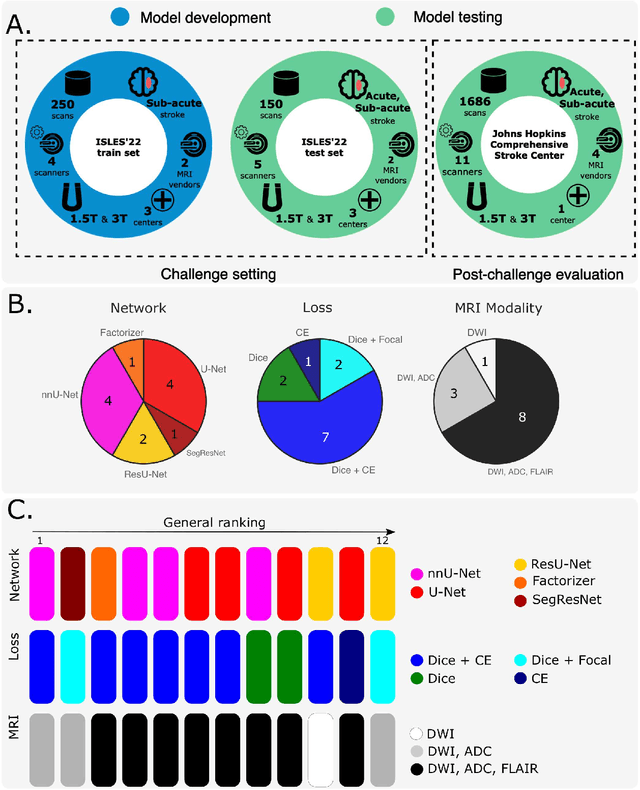
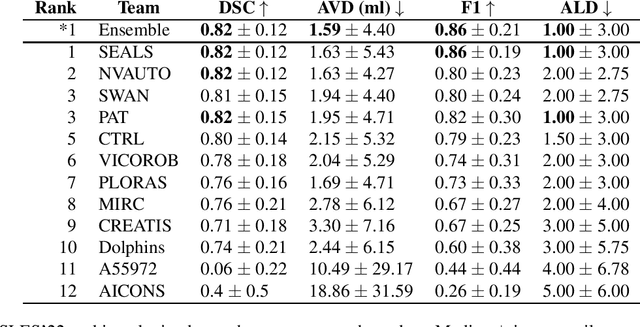
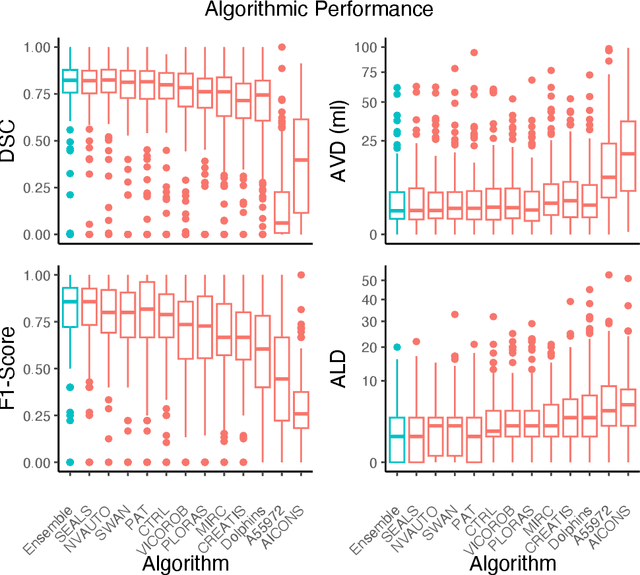
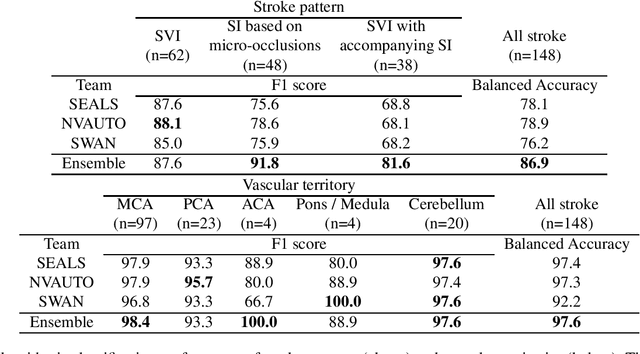
Abstract:Diffusion-weighted MRI (DWI) is essential for stroke diagnosis, treatment decisions, and prognosis. However, image and disease variability hinder the development of generalizable AI algorithms with clinical value. We address this gap by presenting a novel ensemble algorithm derived from the 2022 Ischemic Stroke Lesion Segmentation (ISLES) challenge. ISLES'22 provided 400 patient scans with ischemic stroke from various medical centers, facilitating the development of a wide range of cutting-edge segmentation algorithms by the research community. Through collaboration with leading teams, we combined top-performing algorithms into an ensemble model that overcomes the limitations of individual solutions. Our ensemble model achieved superior ischemic lesion detection and segmentation accuracy on our internal test set compared to individual algorithms. This accuracy generalized well across diverse image and disease variables. Furthermore, the model excelled in extracting clinical biomarkers. Notably, in a Turing-like test, neuroradiologists consistently preferred the algorithm's segmentations over manual expert efforts, highlighting increased comprehensiveness and precision. Validation using a real-world external dataset (N=1686) confirmed the model's generalizability. The algorithm's outputs also demonstrated strong correlations with clinical scores (admission NIHSS and 90-day mRS) on par with or exceeding expert-derived results, underlining its clinical relevance. This study offers two key findings. First, we present an ensemble algorithm (https://github.com/Tabrisrei/ISLES22_Ensemble) that detects and segments ischemic stroke lesions on DWI across diverse scenarios on par with expert (neuro)radiologists. Second, we show the potential for biomedical challenge outputs to extend beyond the challenge's initial objectives, demonstrating their real-world clinical applicability.
Synthetic Data for Robust Stroke Segmentation
Apr 02, 2024Abstract:Deep learning-based semantic segmentation in neuroimaging currently requires high-resolution scans and extensive annotated datasets, posing significant barriers to clinical applicability. We present a novel synthetic framework for the task of lesion segmentation, extending the capabilities of the established SynthSeg approach to accommodate large heterogeneous pathologies with lesion-specific augmentation strategies. Our method trains deep learning models, demonstrated here with the UNet architecture, using label maps derived from healthy and stroke datasets, facilitating the segmentation of both healthy tissue and pathological lesions without sequence-specific training data. Evaluated against in-domain and out-of-domain (OOD) datasets, our framework demonstrates robust performance, rivaling current methods within the training domain and significantly outperforming them on OOD data. This contribution holds promise for advancing medical imaging analysis in clinical settings, especially for stroke pathology, by enabling reliable segmentation across varied imaging sequences with reduced dependency on large annotated corpora. Code and weights available at https://github.com/liamchalcroft/SynthStroke.
Large-kernel Attention for Efficient and Robust Brain Lesion Segmentation
Aug 14, 2023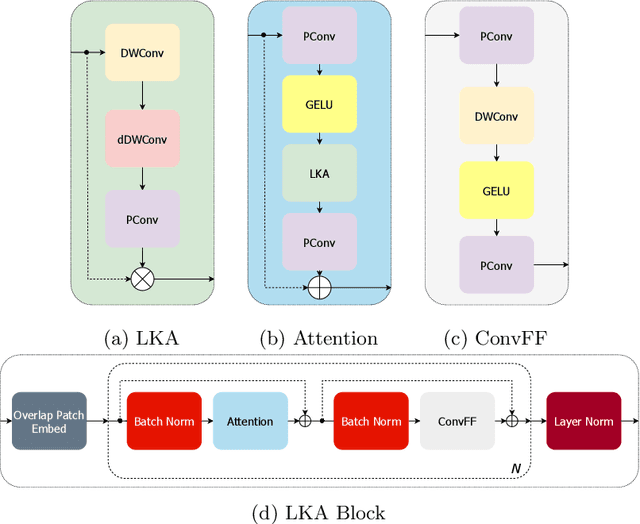
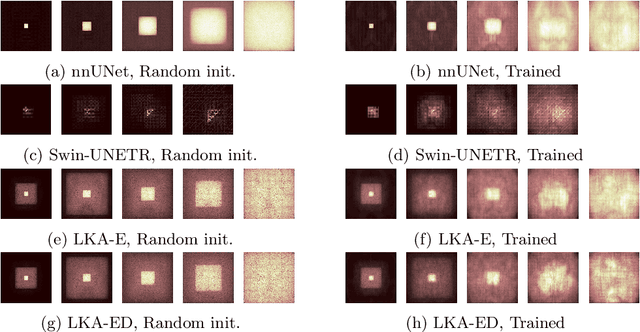
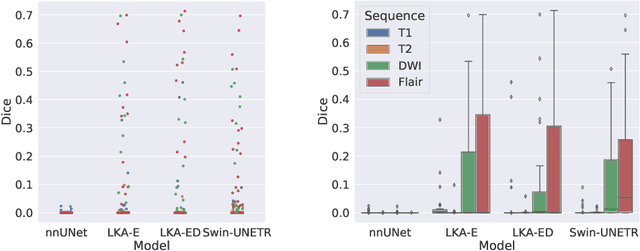
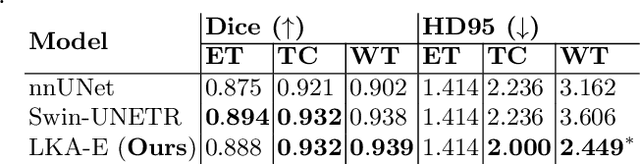
Abstract:Vision transformers are effective deep learning models for vision tasks, including medical image segmentation. However, they lack efficiency and translational invariance, unlike convolutional neural networks (CNNs). To model long-range interactions in 3D brain lesion segmentation, we propose an all-convolutional transformer block variant of the U-Net architecture. We demonstrate that our model provides the greatest compromise in three factors: performance competitive with the state-of-the-art; parameter efficiency of a CNN; and the favourable inductive biases of a transformer. Our public implementation is available at https://github.com/liamchalcroft/MDUNet .
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge