Idowu Paul Okuwobi
Automated Quantification of Hyperreflective Foci in SD-OCT With Diabetic Retinopathy
Jul 31, 2024



Abstract:The presence of hyperreflective foci (HFs) is related to retinal disease progression, and the quantity has proven to be a prognostic factor of visual and anatomical outcome in various retinal diseases. However, lack of efficient quantitative tools for evaluating the HFs has deprived ophthalmologist of assessing the volume of HFs. For this reason, we propose an automated quantification algorithm to segment and quantify HFs in spectral domain optical coherence tomography (SD-OCT). The proposed algorithm consists of two parallel processes namely: region of interest (ROI) generation and HFs estimation. To generate the ROI, we use morphological reconstruction to obtain the reconstructed image and histogram constructed for data distributions and clustering. In parallel, we estimate the HFs by extracting the extremal regions from the connected regions obtained from a component tree. Finally, both the ROI and the HFs estimation process are merged to obtain the segmented HFs. The proposed algorithm was tested on 40 3D SD-OCT volumes from 40 patients diagnosed with non-proliferative diabetic retinopathy (NPDR), proliferative diabetic retinopathy (PDR), and diabetic macular edema (DME). The average dice similarity coefficient (DSC) and correlation coefficient (r) are 69.70%, 0.99 for NPDR, 70.31%, 0.99 for PDR, and 71.30%, 0.99 for DME, respectively. The proposed algorithm can provide ophthalmologist with good HFs quantitative information, such as volume, size, and location of the HFs.
* IEEE Journal of Biomedical and Health Informatics, Volume: 24, Issue: 4, pp. 1125 - 1136, 2020
Can autism be diagnosed with AI?
Jun 05, 2022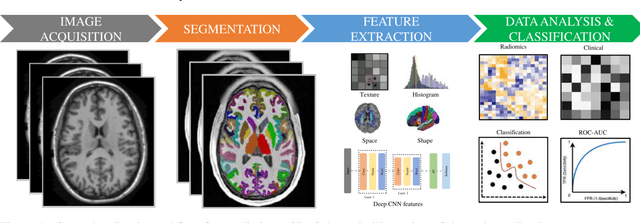
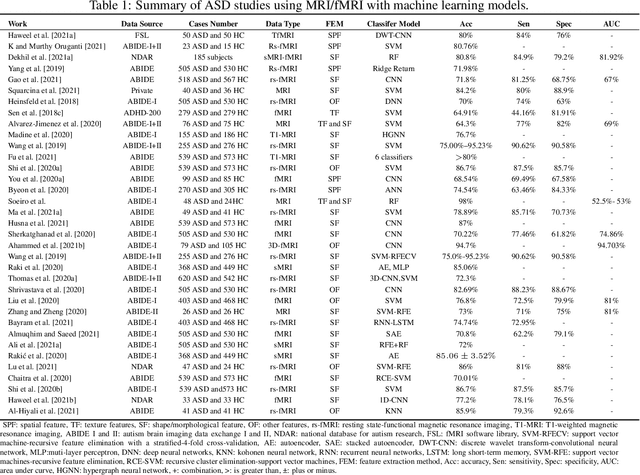
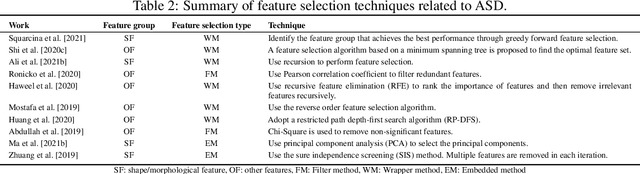
Abstract:Radiomics with deep learning models have become popular in computer-aided diagnosis and have outperformed human experts on many clinical tasks. Specifically, radiomic models based on artificial intelligence (AI) are using medical data (i.e., images, molecular data, clinical variables, etc.) for predicting clinical tasks like Autism Spectrum Disorder (ASD). In this review, we summarized and discussed the radiomic techniques used for ASD analysis. Currently, the limited radiomic work of ASD is related to variation of morphological features of brain thickness that is different from texture analysis. These techniques are based on imaging shape features that can be used with predictive models for predicting ASD. This review explores the progress of ASD-based radiomics with a brief description of ASD and the current non-invasive technique used to classify between ASD and Healthy Control (HC) subjects. With AI, new radiomic models using the deep learning techniques will be also described. To consider the texture analysis with deep CNNs, more investigations are suggested to be integrated with additional validation steps on various MRI sites.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge